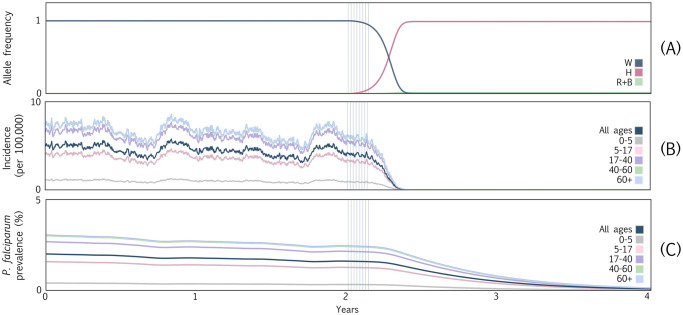
Fig 3. Example MGDrivE 3 simulations for a full gene drive system designed to drive dual malaria-refractory genes into an An. coluzzii mosquito population with seasonal population dynamics, transmission intensity and interventions calibrated to a setting resembling the island of São Tomé, São Tomé and Príncipe.
The gene drive system resembles one recently engineered in An. coluzzii [2] in which all drive components—the Cas9, guide RNA and effector genes—are all present at the same locus. Four alleles are considered: an intact drive allele (denoted by “H”), a wild-type allele (denoted by “W”), a functional, cost-free resistant allele (denoted by “R”), and a non-functional or otherwise costly resistant allele (denoted by “B”). Model parameters describing the construct, mosquito bionomics and malaria transmission are summarized in S1 Table. (A) Allele frequencies for adult female mosquitoes over the simulation period. Grey vertical bars beginning at year two denote eight consecutive weekly releases of 20,000 male mosquitoes homozygous for both the gene drive construct. The high efficiency of the drive system and low rate of resistance allele generation mean that almost no disease-competent An. coluzzii mosquitoes remain five months after the release. (B) Daily clinical malaria incidence per 100,000 people partitioned according to age group. Reductions in human incidence within five months of the release parallel spread of the drive construct in the mosquito population. (C) P. falciparum malaria prevalence partitioned according to age group. As humans recover from infection and few develop new infections, the P. falciparum parasite rate declines until it reaches near undetectable levels by year five.