Abstract
The tibial fracture-pin model is a mouse model of orthopedic trauma and surgery that recapitulates the complex muscle, bone, nerve, and connective tissue damage that manifests with this type of injury in humans. This model was developed because previous models of orthopedic trauma did not include simultaneous injury to multiple tissue types (bone, muscle, nerves) and were not truly representative of human complex orthopedic trauma. The authors therefore modified previous models of orthopedic trauma and developed the tibial fracture-pin model. This modified fracture model consists of a unilateral open tibial fracture with intramedullary nail (IMN) internal fixation and simultaneous tibialis anterior (TA) muscle injury, resulting in mechanical allodynia that lasts up to 5 weeks post injury. This series of protocols outlines the detailed steps to perform the clinically relevant orthopedic trauma tibial fracture-pin model, followed by a modified hot plate assay to examine nociceptive changes after orthopedic injury. Taken together, these detailed, reproducible protocols will allow pain researchers to expand their toolkit for studying orthopedic trauma-induced pain.
Introduction
Orthopedic trauma accounts for 25% of all injuries sustained by nearly 500 million people each year worldwide1,2,3. Orthopedic trauma can be associated with complex muscle, bone, nerve, and connective tissue damage, necessitating hospitalization and surgery to ensure adequate recovery3,4. Acute and chronic pain after orthopedic trauma can result in significant physical, psychological, and financial burdens that affect a patient’s quality of life1,4. Additionally, orthopedic surgery to stabilize and fix fractures is also associated with severe acute and chronic post-surgical pain5,6,7,8,9.
The mechanisms underlying acute and chronic trauma-related pain need to be better understood to develop better treatments. To achieve this, reliable, reproducible, and clinically relevant preclinical models are required. Since most animal models of orthopedic trauma did not involve simultaneous injury to multiple tissue types (bone, muscle, nerves), they were not truly representative of human complex orthopedic trauma, for example, trauma after falls, motor vehicle crashes, or war-related injuries10,11. Therefore, we developed the tibial fracture-pin mouse model to examine the major manifestations of such injury, including bone and muscle tissue damage and acute and chronic pain11. The tibial fracture-pin model consists of a unilateral open tibial fracture with IMN internal fixation and simultaneous TA muscle injury. Histological sections of the TA show injury to the muscle in which dense fibrosis develops with associated loss of large, mature muscle fibers as early as 2 weeks post injury. Moreover, the fracture callus is apparent on microcomputer tomography (microCT) 4 weeks post injury and continues to undergo remodeling11.
Various reflexive and nonreflexive behavior assays can be used to evaluate the sensory and affective components of pain in the tibial fracture-pin model. For example, one can use the Von Frey filaments to demonstrate mechanical hypersensitivity in this model. In fact, mice develop long-lasting mechanical hypersensitivity in the ipsilateral hind paw after tibial fracture-pin surgery11. Another particularly useful behavioral paradigm is the hot plate assay, which traditionally measures the latency to paw withdrawal to a thermal stimulus. While this assay has been used for decades12, truly a gold standard in preclinical pain research, measuring reflexive behavior alone has its limitations13. As a result, we have developed a modified hot plate paradigm that can capture elements of both reflexive and nonreflexive responses in the setting of a thermal stimulus14.
This modified hot plate assay determines the initial response latency as in the original hot plate test and an extended observation period to record additional nocifensive behaviors. By categorizing these extended behaviors into distinct categories (flinching, licking, guarding, jumping), the nonreflexive response to the thermal stimulus can be captured. Flinching is the rapid removal of the paw and/or splaying of digits, but the limb is quickly returned to the hot plate. Licking and biting of the hind and front paws are both defined as licking for analysis. Guarding is the continued raising of the limb beyond when afferent nociceptive information ends. Finally, jumping is the removal of all four limbs from the hot plate surface. These behaviors can be analyzed individually and grouped together with special care to still note the initial response latency.
Protocol
All methods used while conducting this research were performed in compliance and with approval by the Stanford University Administrative Panel on Laboratory Animal Care (APLAC #33114) in accordance with American Veterinary Medical Association guidelines and the International Association for the Study of Pain. Mice (C57BL/6J, 9–11 weeks old upon arrival, 11–12 weeks old at study initiation) were housed 2–5 per cage and maintained on a 12 h light/dark cycle in a temperature-controlled environment with ad libitum access to food and water. Male mice weighed approximately 25 g at the start of the study. See the Table of Materials for details regarding all materials used in this study.
Materials.
1. Baseline behavior measurements
As mice learn quickly on the hot plate assay, do not record a baseline for mice in this assay. Instead, compare the mice to uninjured controls.
2. Anesthesia/preparation
Anesthetize the mouse with inhalational isoflurane 2%–4%.
Pinch the toe with forceps and use the loss of the toe pinch reflex to confirm the depth of anesthesia.
Apply eye lubricant generously to the mouse’s eyes to prevent dryness while under anesthesia.
Place a piece of gauze under the mouse and use clippers to remove hair from the right leg of the mouse up to the knee joint.
Clean the hair from the surgical area using the gauze, and then discard the gauze.
Disinfect the surgical area using a cotton swab dipped in iodine solution.
3. Surgery
After cleaning the surgical area and confirming the depth of anesthesia, use a scalpel to make a skin incision on the medial surface of the right leg from the distal tibia to the proximal tibia, stopping at the level of the inferior knee joint (Figure 1A).
Dry the area using a cotton swab with particular attention to the proximal tibia.
Using a drill with a 0.6 mm round burr drill bit, drill a hole at the proximal end of the tibia at the level of the tibial tuberosity, ~2 mm distal to the joint line (Figure 1B).
Then, insert a 27 G needle through this hole/osteotomy down the medullary axis of the bone to establish a channel, and then remove the needle (Figure 1B).
-
Next, use a bone saw to score the tibia at the junction of the middle and distal thirds (~5–6 mm distal to the osteotomy site) from the lateral aspect causing trauma to the tibialis anterior muscle (Figure 1D).
CRITICAL: A medial approach to the fracture will not produce muscle injury.
Clamp the proximal end of the tibia, hold the distal end of the tibia between the thumb and forefinger, and use counter pressure to complete the bone fracture (Figure 1C).
-
Reinsert the 27 G needle into the intramedullary space, through the proximal tibia, and advance it across the fracture site to the distal segment of the bone to align the fracture.
NOTE: A similarly sized ceramic implant may be inserted instead of a 27 G needle in imaging applications where metal is undesirable. Although not performed here, a possible implant to consider is a ceramic screw (see the Table of Materials).
Then, cut the needle/plastic implant flush with the tibial cortex using cutting pliers.
Clean up any blood and confirm that the bleeding has stopped before proceeding.
-
Once bleeding has stopped, close the wound using an interrupted 5–0 silk suture.
CRITICAL: Do not leave the animal unattended at any point in the surgery. Observe the animal until it is able to mobilize independently and return it to its cagemates only once it has fully recovered.
Figure 1: The orthopedic injury model involving unilateral tibial fracture and resulting in muscle fibrosis.
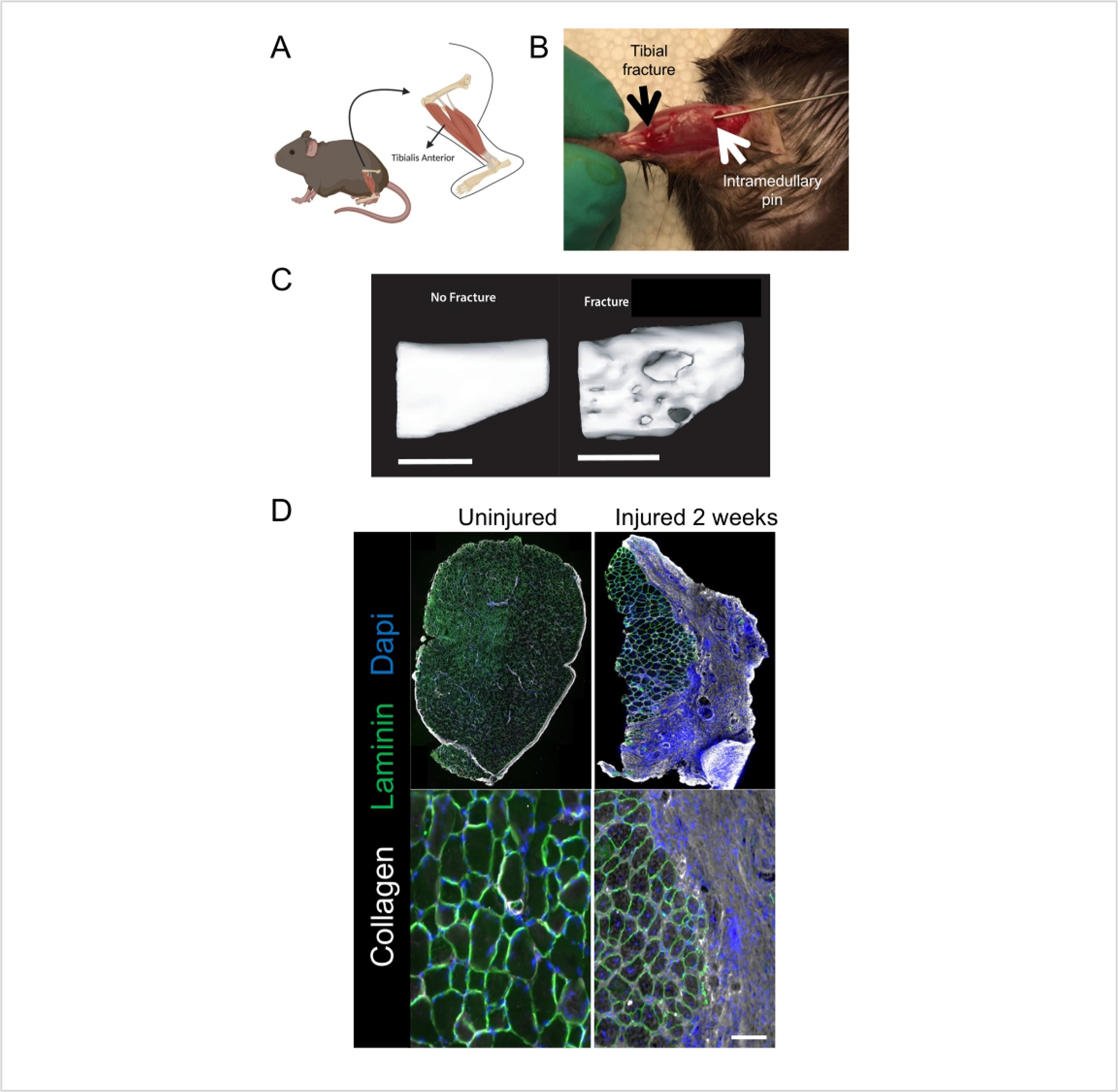
(A) Schematic of the location of the tibia and tibialis anterior muscle, which are both injured in this model. (B) Intraoperative photograph demonstrating location of tibial bone fracture (black arrow), osteotomy and intramedullary nail (white arrow), and TA muscle. (C) microCT scan of the right tibia without fracture (left) or 4 weeks after fracture (right), demonstrating clear callus formation. (D) Orthotrauma results in extensive fibrosis of the TA muscle, as demonstrated by immunohistochemical staining of TA muscle sections showing increased collagen (white) and altered muscle fiber pattern (laminin, green) as well as loss of regular, central nuclei (DAPI, blue). Scale bars = 2 mm (C), 100 μm (D). This figure was adapted, with permission, from Tawfik et al. 202011. Abbreviations: TA = tibialis anterior; microCT = Micro Computer Tomography; DAPI = 4’,6-diamidino-2-phenylindole.
4. After surgery
After surgery, administer buprenorphine 0.05 mg kg−1 by subcutaneous injection to the mice twice daily for two days per local protocols.
Monitor the mice in the post-anesthesia period and for the length of the study as follows: twice daily for the first 2 days, and then daily up to 3 weeks, and weekly thereafter.
-
Assess the mice for behavioral changes indicative of severe stress or sickness such as lethargy, ruffled hair coat, or >20% weight loss. Take note of any issues related to mobility and access to food or water.
NOTE: Surgical and post-care logs must be filled after surgery and at each check and maintained indefinitely for Institutional Animal Care and Use Committee auditing.
5. Hot plate testing
NOTE: Postinjury measurements can begin 7 days after tibial fracture-pin surgery. To avoid the effect of learning in this paradigm, perform the test once after surgery and compare to uninjured controls.
- Setup
- Place the hot plate in a configuration where the lighting is overhead, and the plastic cylinder is centered on the plate.
-
Set the temperature to 50 °C and place a camera in front of the plate with care to keep the entirety of the enclosed area in view.CRITICAL: A standard 8-bit color industrial camera, which records up to 76 fps, was used in this study.
- Assay
-
Place the mice inside the testing room for at least 30 min to habituate.NOTE: Make sure the testing room is not too hot or cold to avoid skewing the results.
- Place the mouse in the cylinder and begin recording.
- Record movements for 45 s after the mouse’s feet first make contact.
- Repeat steps 5.2.1–5.2.3 with the rest of the cohort while maintaining temperature for each subsequent trial.
-
Measure latency (in seconds) to first response (usually flinching), which is the classic hot plate outcome.NOTE: The following protocol developed in this laboratory additionally scores both reflexive (flinching) and nonreflexive (licking, guarding, and jumping).
-
- Analysis
- Score the hot plate sessions using a viewing program that provides a time stamp with milliseconds.
- Consider using NCH Prism Software on a Mac OS. Download this software for free online (see Figure 2 and Table of Materials).
- Once downloaded, open the program and click on the option to Continue to Use the Demo Version. Next, click on the large green plus sign to upload the hot plate recordings.
-
Once uploaded, double-click on an individual file to open in video format. Utilize the cursor at the bottom of the window to slowly drag through the length of the video and begin scoring.CRITICAL: Regardless of the software used, make sure to watch the videos in the largest window possible to avoid losing time resolution when dragging the time stamp cursor through the video.
- Format a spreadsheet as follows.
- To properly run the R scripts provided, write the column titles exactly as listed below with no capitalization or spaces and in the order listed: mouseid, behavior, start, end.
- Enter the data into the start and end columns in time in s to three decimal places with no leading zeros or colons (e.g.: 2.001)
- For each video, record each instance of a pain behavior (to ms). Note that only instances of pain behavior by the two hind paws are recorded. Do not record the behavior of the two front paws.
- Record flinching/flicking simply as “flinching,” including rapid withdrawal of the paw and/or splaying of digits, but the limb is quickly returned to the hot plate, so long as they are not exploring/walking. Count splaying of the digits without actually lifting the entire paw also as flinching. Do not record flinching/flicking behavior for longer than 500 ms. If recorded as such, drag the cursor through this behavior as slowly as possible as it is likely that several flinches and/or guarding occurred in this span.
- Look for prolonged elevation/protection of a limb beyond when afferent nociceptive information ends and record it as guarding.
- Record licking/biting of a hind paw as licking.
-
Record the removal of all four limbs from the hot plate at once as jumping.NOTE: If a mouse flinches, and then with the paw up, continues to attend by licking/guarding, and then split the behavior without any time stamp overlap. Create a separate spreadsheet for each experimental group.
- Utilize the provided R scripts to begin analyzing the data.
- Use “Behavior_Raster_v2.R” to generate a raster plot (as shown below) for visualizing the overall behavior.
-
Use “Behavior_duration.R” to write a spreadsheet containing five sheets to the working directory.NOTE: The first sheet provides the total duration of all pain behaviors, the latency to the first pain behavior, and the total number of pain behaviors. Each subsequent sheet provides this information for individual pain behaviors.
- Use “Behavior_bins.R” to write two spreadsheets; one with 500 ms bins showing cumulative duration of behavior at each time point, and the other with the area under the curve for the durational behavior profile of each mouse.
- Finally, use “Cumulative_Sums.R” to write two spreadsheets but for cumulative summation of a behavior.
Figure 2: Set-up for download and use of NCH Prism Software.
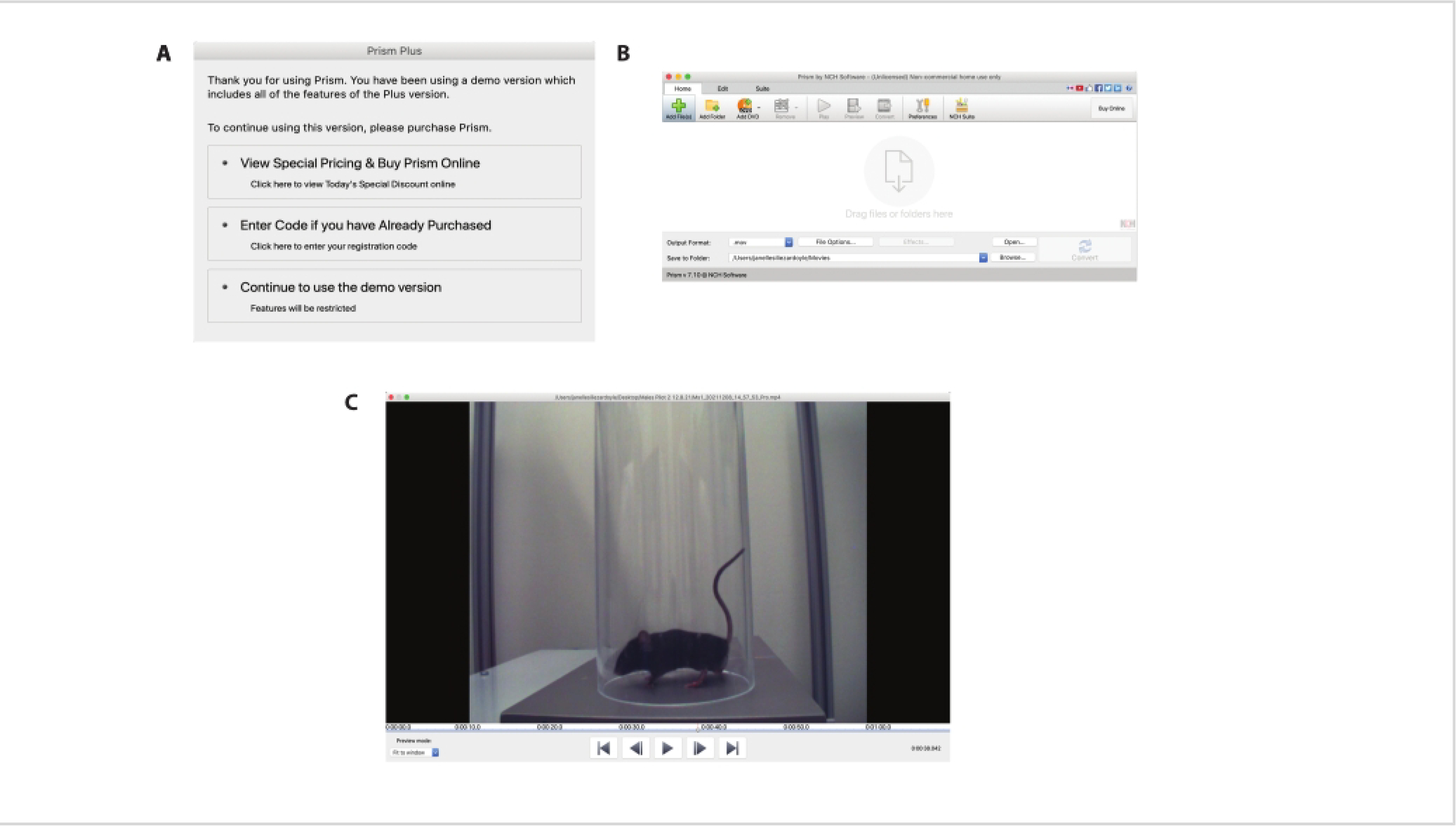
(A) This panel shows a screenshot of the options that are available after opening NCH Prism Software. To utilize the free version, click on Continue to Use the Demo Version. (B) Once the program is fully open, a new window will open (shown here in panel B) where all the videos can be uploaded. Click on the green plus sign to add video files or drag files into the window. Once the videos are uploaded in the NCH Prism, double-click on a single video to open a large viewing window. (C) An example of a video being viewed in the NCH Prism is shown here. Note the millisecond timestamp at the bottom-right corner of the window and the cursor that can be dragged manually through the length of the video.
Representative Results
The tibial fracture-pin orthotrauma model reproduces the bone, muscle, and pain-like behaviors seen in complex human injury. As shown in Figure 1C, the tibial fracture heals over time, forming a callus at the fracture site that is still seen at 4 weeks post injury. As a result of the lateral approach with the bone saw described above (step 3.5), the tibialis anterior muscle is injured, becoming extensively fibrotic, as seen by increased collagen deposition throughout the tissue (Figure 1D). Hind paw sensitivity is quite profound, as evidenced by decreased threshold to mechanical stimuli, lasting at least 5 weeks after injury11. In addition, we have incorporated novel non-reflexive assays to more fully explore pain-like behaviors after peripheral injury14, 15. As shown in Figure 3, orthotrauma injury results in increased reflexive flinching and non-reflexive behaviors (licking, guarding). Note that in some cases, mice also exhibit jumping, which is considered a non-reflexive behavior.
Figure 3: Raster plots showing distinct nocifensive behaviors in an orthotrauma (injured) animal two weeks after injury compared to an uninjured control using a hot plate assay.
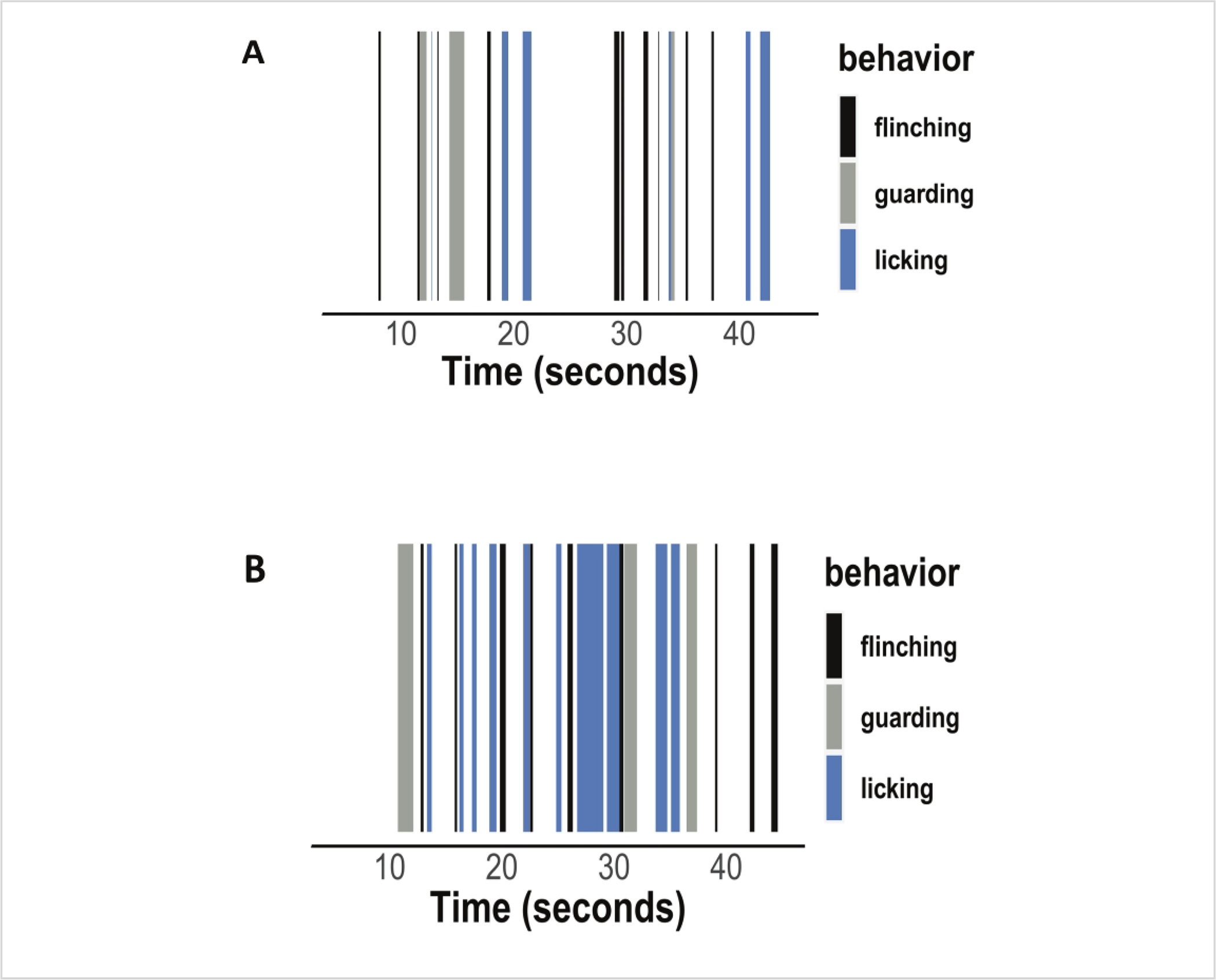
(A) Raster plot showing flinching, guarding, and licking behaviors in a control animal. (B) Raster plot showing increased flinching, guarding, and licking behaviors in an injured animal.
Discussion
Critical steps within the protocol
It is crucial to maintain sterile conditions throughout the surgery. Moreover, proper animal care before, during, and after the surgery is paramount for the successful generation of the model. As mentioned earlier in the protocol, when performing the surgery, fracture the bone from the lateral side to ensure muscle injury. Take care not to fracture the tibia too low (below the advised junction between the middle and distal thirds of the tibia) because this will affect how the bone fractures, the researcher’s ability to fix it with a pin, and the healing of the fracture.
To further ensure the translatability of preclinical findings to clinically useful treatments, the tibial fracture-pin model must be combined with outcome measures that have clear human correlates. Using the modified hot plate assay described above allows for the observance of temperature sensitivity due to chronic pain models in rodents. Individuals with chronic pathological conditions often report increased sensitivity and pain with changes in temperature. Increased temperature can lead to increased inflammation and impact the way that tissues expand and contract. Therefore, all preclinical researchers are encouraged to consider these approaches in designing their studies to evaluate acute and chronic postsurgical pain. One important part of obtaining reliable and reproducible results from the modified hot plate assay is to follow a strict protocol for determining individual behaviors on review of the videos. We have a very protocolized regimen for training new researchers on the video review and require them to meet the desired metrics for interrate reliability prior to having them score videos independently.
Limitations, modifications, and troubleshooting of the technique
Since the protocol described includes the use of a 27 G needle as an implant, one has to modify it to pair it with certain kinds of imaging, which may be adversely affected by a metal implant, for example, computed tomography (CT) or magnetic resonance imaging (MRI). Therefore, for imaging applications, replacing the metal implant with a suitable ceramic or plastic implant is advisable.
We have previously performed the hot plate assay at 52.5 °C and recorded significant differences in latency and nonreflexive behaviors, which were increased after peripheral injury15 and suppressed by morphine14. That said, we have opted to perform these hot plate experiments at a lower temperature of 50 °C going forward to better discriminate more subtle findings that may arise from less severe injuries or less potent analgesics. It is therefore recommended that those adopting this new technique trial multiple temperatures ensure that they do not run into a ceiling effect in their particular model.
Significance with respect to existing methods
Orthopedic injuries involving bone, muscle, and nerve trauma are a major public health concern common in many settings, including falls, sports-related trauma, motor vehicle accidents, and military combat11. Preclinical researchers need mouse models of orthopedic trauma that mimic multi-tissue injury, including bone, muscle, and nerve, since they are most representative of human orthopedic trauma. The tibial fracture-pin model was created to meet this need. Indeed, it has been shown that this model reproduces many features of orthopedic trauma seen in humans11.
Any future applications of the technique
In addition to studying mechanisms of trauma-induced pain, the fracture-pin model can also be used to evaluate drug candidates for orthopedic pain treatment. It can also be used to study fracture healing and evaluate the effects of different candidate therapeutics for fracture healing.
Acknowledgments
GM is supported by an NDSEG Graduate Fellowship and a Stanford Bio-X Honorary Graduate Fellowship. VLT is supported by NIH NIGMS grant #GM137906 and the Rita Allen Foundation.
Footnotes
A complete version of this article that includes the video component is available at http://dx.doi.org/10.3791/63590.
The authors have no relevant conflicts of interest to disclose.
References
- 1.Edgley C et al. Severe acute pain and persistent post-surgical pain in orthopaedic trauma patients: a cohort study. British Journal of Anaesthesia. 123 (3), 350–359 (2019). [DOI] [PubMed] [Google Scholar]
- 2.Pointer S Trends in Hospitalised Injury, Australia: 1999–00 to 2010–11. Australian Institute of Health and Welfare; (2013). [Google Scholar]
- 3.James SL et al. Global, regional, and national incidence, prevalence, and years lived with disability for 354 diseases and injuries for 195 countries and territories, 1990–2017: a systematic analysis for the Global Burden of Disease Study 2017. The Lancet. 392 (10159), 1789–1858 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shen H, Gardner AM, Vyas J, Ishida R, Tawfik VL Modeling complex orthopedic trauma in rodents: Bone, muscle and nerve injury and healing. Frontiers in Pharmacology. 11, 620485 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gerbershagen HJ et al. Pain intensity on the first day after surgery: A prospective cohort study comparing 179 surgical procedures. Anesthesiology. 118 (4), 934–944 (2013). [DOI] [PubMed] [Google Scholar]
- 6.Clay FJ, Watson WL, Newstead SV, McClure RJ A systematic review of early prognostic factors for persisting pain following acute orthopedic trauma. Pain Research and Management. 17 (1), 35–44 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Williamson OD et al. Predictors of moderate or severe pain 6 months after orthopaedic injury: A prospective cohort study. Journal of Orthopaedic Trauma. 23 (2), 139–144 (2009). [DOI] [PubMed] [Google Scholar]
- 8.Friesgaard KD et al. Persistent pain is common 1 year after ankle and wrist fracture surgery: a register-based questionnaire study. British Journal of Anaesthesia. 116 (5), 655–661 (2016). [DOI] [PubMed] [Google Scholar]
- 9.Holmes A et al. Predictors of pain severity 3 months after serious injury. Pain Medicine. 11 (7), 990–1000 (2010). [DOI] [PubMed] [Google Scholar]
- 10.Guo T-Z et al. Immobilization contributes to exaggerated neuropeptide signaling, inflammatory changes, and nociceptive sensitization after fracture in rats. The Journal of Pain. 15 (10), 1033–1045 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tawfik VL et al. Angiotensin receptor blockade mimics the effect of exercise on recovery after orthopaedic trauma by decreasing pain and improving muscle regeneration. The Journal of Physiology. 598 (2), 317–329 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Deuis JR, Dvorakova LS, Vetter I Methods used to evaluate pain behaviors in rodents. Frontiers in Molecular Neuroscience. 10, 284 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Porreca F, Navratilova E Reward, motivation, and emotion of pain and its relief. Pain. 158 Suppl 1 (Suppl 1), S43–S49 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Corder GF et al. Loss of mu-opioid receptor signaling in nociceptors, and not spinal microglia, abrogates morphine tolerance without disrupting analgesic efficacy. Nature Medicine. 23 (2), 164–173 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Huck NA et al. Temporal contribution of myeloid-lineage TLR4 to the transition to chronic pain: A focus on sex differences. Journal of Neuroscience. 41 (19), 4349–4365 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]


