Abstract
The modified vaccinia virus Ankara (MVA) strain is a candidate vector for vaccination against pathogens and tumors, due to safety concerns and the proven ability of recombinants based on this vector to trigger protection against pathogens in animals. In this study we addressed the fate of the MVA vector in BALB/c mice after intraperitoneal inoculation in comparison with that of the replication-competent Western Reserve (WR) strain by measuring levels of expression of the reporter luciferase gene, the capability to infect target tissues from the site of inoculation, and the length of time of virus persistence. We evaluated the extent of humoral and cellular immune responses induced against the virus antigens and a recombinant product (β-galactosidase). We found that MVA infects the same target tissues as the WR strain; surprisingly, within 6 h postinoculation the levels of expression of antigens were higher in tissues from MVA-infected mice than in tissues from mice infected with wild-type virus but at later times postinoculation were 2 to 4 log units higher in tissues from WR-infected mice. In spite of this, antibodies and cellular immune responses to viral vector antigens were considerably lower in MVA-inoculated mice than in WR virus-inoculated mice. In contrast, the cellular immune response to a foreign antigen expressed from MVA was similar to and even higher than that triggered by the recombinant WR virus. MVA elicited a Th1 type of immune response, and the main proinflammatory cytokines induced were interleukin-6 and tumor necrosis factor alpha. Our findings have defined the biological characteristics of MVA infection in tissues and the immune parameters activated in the course of virus infection. These results are of significance with respect to optimal use of MVA as a vaccine.
The successful use of vaccinia virus (VV) during the worldwide program for eradication of smallpox (57), in conjunction with the development of strategies to generate recombinant VV (rVV) efficiently expressing foreign proteins (31, 39), has increased the potential use of poxvirus-derived vectors as delivery systems in vaccination programs. Moreover, the advent of the AIDS pandemic has focused attention on live-virus-based vaccines, as both humoral immunity and cell-mediated immunity (CMI) are generated during replication of the viral vector. Cellular immunity seems to be relevant in the development of protection against human immunodeficiency virus type 1 (HIV-1) (45) and simian immunodeficiency virus (SIV) (25, 48), representing the immunological parameter that correlates with protection against disease. Because poxvirus-derived vectors activate both branches of the immune system (14), they are becoming promising vectors in efforts to develop an efficient anti-HIV vaccine, with economy of production and easy worldwide distribution. However, rare but grave nondesired side effects due to complications associated with vaccination with VV, increased in immunosuppressed individuals (19, 41), represent serious difficulties for wide use of VV-derived vaccines. All of these factors have prompted the development of highly attenuated VV-derived viruses that can be used for safe vaccines if the desired immunological properties are maintained while virulent genes are removed from the viral genome.
Avipoxvirus-derived vectors (55) and highly attenuated strains of VV have been considered candidate vectors (37, 42). The modified vaccinia virus Ankara (MVA), derived from the Ankara VV strain through more than 500 passages in chicken embryo fibroblasts (CEFs), was used in almost 120,000 Caucasian individuals with no reported side effects, although many of the subjects were among the population with high risk of developing complications (33). Genetic analysis showed that during attenuation, viral genes spanning 15% of the parental genome (ca. 30 kbp) were lost (34); this loss included naturally encoded VV genes involved in host immunoregulation (8) and host range genes (1, 34). The MVA genome has been sequenced, and the genes lost during passage have been identified (2). Among the main properties that encourage the future use of MVA as a vaccine vector for humans are previous field experience and the inability of the virus to replicate productively in human cell lines and primary human cell cultures (17). The defect in the viral life cycle is in the final steps of the morphogenetic program, with no alteration in early or late virus gene expression or in the levels of foreign protein expression, which in cultured cells is at least as efficient as that for other, more virulent VV strains (53).
Different viral and tumor animal models (11) have been used to demonstrate the efficacy of MVA recombinants in the development of protective immunity. Thus, immunity to influenza virus and parainfluenza virus type 3 was achieved after intramuscular and intranasal inoculation of recombinant MVA (rMVA) expressing hemagglutinin and nucleoproteins in murine models (54, 59), and protection against the latter was observed in rhesus monkeys (18). In the SIV model system, env gene expression by MVA in macaques conferred partial immunity (23); more recently, mouse models have demonstrated that MVA is an efficient vector for development of mucosal immunity against HIV antigens (6). This vector has also proven to be efficient as a booster during DNA immunization schemes designed to enhance the immune response against HIV epitopes (21), a property that was previously demonstrated for the virulent WR strain in the malaria model system (49).
However, despite the use of MVA in the mouse model system as a first step in vaccine research, no systematic studies have been carried out in vivo either on the virus life cycle or on immunological parameters triggered in the course of virus infection. Knowledge of these basic biological features of MVA infection will be useful for improving immunization procedures based on MVA and will help to define immune parameters that could affect the further success of vaccination strategies.
In this study, we have addressed fundamental issues related to the potential use of MVA as a vaccine. We have characterized the kinetics of expression of MVA-encoded genes in different tissues, the nature of the immune response triggered to the MVA vector, and the effectiveness of the immune response against a recombinant antigen in comparison with the laboratory strain WR (Western Reserve). Our findings demonstrate that MVA expresses in vivo foreign antigens transiently and, compared with the WR strain, elicits a lower immune response against itself while eliciting a similar or even higher immune response against a foreign antigen. The cytokines activated in response to MVA have been characterized and found to be clearly biased toward a Th1-type pattern. Proinflammatory cytokines (interleukin-6 [IL-6] and tumor necrosis factor alpha [TNF-α]) were higher in MVA-infected mice than in wild-type-infected animals. These findings are relevant to MVA vaccination strategies in which more than one immunization dose might be required, as the immune response against the vector is low and the immune response to the recombinant antigen might be increased with repeated boosters or with combination of vectors.
MATERIALS AND METHODS
Viruses and cells.
VV recombinants used in this study were derived from either the MVA (kindly provided by G. Sutter, Munich, Germany) or WR strain. MVAluc and WRluc, expressing the luciferase and β-galactosidase (β-Gal) genes inserted in the thymine kinase gene, were generated according to standard methods; the latter virus has been described previously (43). MVAluc was grown in BHK-21 cells and plaque purified during six passages. WRluc was grown in HeLa cells, titrated in African green monkey kidney BSC-40 cells, and purified as described previously (15). Purity of stocks of both viruses was checked by PCR using oligonucleotides complementary to the thymidine kinase sequences. CEFs were obtained from sterile pathogen-free eggs; cultures were obtained according to standard procedures and maintained in Dulbecco modified Eagle medium supplemented with 10% fetal calf serum (FCS), in the same way as the baby hamster kidney cell line BHK-21. NIH 3T3 cells were maintained in Dulbecco modified Eagle medium supplemented with 5% calf serum.
MVA virus titration.
We have analyzed different procedures for titrating MVA in an attempt to select a simple and reliable method which guarantees that infectious units are being counted. Purified viral stocks of MVAluc and WRluc were titrated on BHK-21 and CEF monolayers by plaque assay and by immunostaining of fixed infected cultures with polyclonal serum reactive against VV proteins. For example, WRluc viral stock titers were (8.5 ± 1.7) × 109 PFU/ml or (8.7 ± 1.5) × 109 immunospots/ml; titers were (3.0 ± 0.5) × 109 PFU/ml and (2.7 ± 0.2) × 109 immunospots/ml for MVAluc virus stock. Comparable results were obtained with WRluc when assayed on BSC-40 cells (data not shown). As the visualization of MVA plaques is sometimes arduous, the results described allowed us to establish the immunostain method with polyclonal anti-VV serum as a reliable index of the number of infectious units present in viral stocks and to assume that it corresponds to PFU.
Immunizations of mice and serum sample collection.
BALB/c mice (H-2d) (6 to 8 weeks old) were immunized intraperitoneally (i.p.) with different doses (indicated as PFU) of WRluc or MVAluc virus in 200 μl of sterile phosphate-buffered saline (PBS). Fourteen days after virus inoculation, blood was obtained from the retro-orbital plexus by a capillary tube, collected in an Eppendorf tube, and centrifuged; serum was obtained and stored at −20°C.
Measurement of luciferase activity in mouse tissues.
Gene expression of recombinant viruses in different mouse tissues was monitored by a highly sensitive luciferase assay previously described (43). Different groups of mice received an i.p. inoculation of rMVAluc or rWRluc. At various times postinoculation, animals were sacrificed, and spleens, livers, and ovaries were resected, washed with sterile PBS, and stored at −70°C. Then, tissues from individual mice were homogenized in luciferase extraction buffer (300 μl/spleen or liver extract and 100 μl/ovary extract) (Promega Corp., Madison, Wis.). Luciferase activity was measured in the presence of luciferin and ATP according to the manufacturer's instructions, using a Lumat LB 9501 luminometer (Berthold, Nashua, N.H.), and was expressed as luciferase reference units per milligram of protein. Protein content in tissue extracts was measured with a bicinchoninic protein assay reagent kit (Pierce Co., Rockford, Ill.).
β-Gal measurement.
β-Gal activity in infected cell extracts was measured in cleared supernatants from scraped cell cultures, using colorimetric determination with chlorophenol red-β-d-galactopyranoside (Roche Pharmaceuticals, Nutley, N.J.) as instructed by the manufacturer. Triplicate measurements of diluted samples were carried out. Mock-infected cell extracts were used for background levels. Commercial β-Gal (Sigma, St. Louis, Mo.) was used to draw a standard curve and convert absorbance values to units of β-Gal according to the manufacturer's data (520 U of β-Gal/mg).
Neutralization assay.
Sera from immunized mice were inactivated at 56°C for 30 min, and serial dilutions in PBS supplemented with 2% FCS were incubated with 200 PFU of WR virus at 37°C for 1 h. Afterwards, confluent BSC-40 cells monolayers were infected in triplicate, and plaques were visualized 48 h postinoculation (hpi) by crystal violet staining and counted. As a control, inactivated serum from mock-infected mice was used. The number of plaques obtained with each serum was normalized to this control value and used to calculate the neutralization titer.
Immunohistochemistry.
Organs were aseptically removed and fixed in 10% formalin solution (Sigma), embedded in paraffin, and sectioned according to standard procedures with a Microtome Jung RM 2155 (Leica, Cambridge, United Kingdom). For immunostaining, 5-μm-thick sections were deparaffinized, hydrated, incubated with hyperimmune rabbit anti-VV sera at a dilution of 1/500 and then with an ImmunoPure Elite ABC peroxidase staining kit (Pierce) as instructed by the manufacturer, and developed with 3′,3′-diaminobenzidine tetrahydrochloride (DAB; Sigma). After development, slices were counterstained with hematoxylin-eosin and visualized in a Leica DMRXA microscope. Images were captured with the DC100 imaging system from Leica.
Antibody measurements by ELISA.
An enzyme-linked immunosorbent assay (ELISA) was used to determine the presence of antibodies against VV antigens in serum samples. The VV antigens used to coat 96-well flat-bottom plates at a concentration of 1 μg/ml consisted of envelope proteins extracted from purified virions as described previously (44). VV antigens were suspended in carbonate buffer (pH 9.6), plated at 50 μl/well, and incubated overnight at 4°C. Afterwards, contents of the wells were removed and washed three times with PBS plus 0.05% Tween 20 (PBS-T); blocking buffer (borate-buffered saline with 1% bovine serum albumin [BSA], 1 mM EDTA, 0.05% and Tween 20) was added at 100 μl/well, and the plates were incubated for 1 h at 37°C. The plates were washed once with PBS-T, and samples diluted in blocking buffer were added in a volume of 100 μl/well and incubated for 1 h at 37°C. Plates were washed three times before the detection antibody was added. Peroxidase-conjugated goat anti-mouse immunoglobulin G (IgG), IgG1, or IgG2a (Southern Biotechnology Associates, Birmingham, Ala.) antibodies were diluted 1:1,000, 1:1,500, or 1:2,000, respectively, in blocking buffer and incubated for 1 h at 37°C. After the plates were washed three times with PBS-T, the wells were reacted with the peroxidase substrate o-phenylenediamine dihydrochloride (Sigma). After 10 to 15 min of incubation at room temperature, the reaction was stopped by adding 2 N H2SO4, and absorbance was measured at 492 nm on a Multiskan Plus plate reader (Labsystems, Chicago, Ill.).
T-cell proliferation assays.
Lymphocytes were removed from spleens by passing tissues through a sterile mesh to obtain cell suspensions. Cells were suspended in complete medium (RPMI 1640 supplemented with 10% FCS, 2 mM l-glutamine, and 10 μM 2-mercaptoethanol). Erythrocytes in preparations of spleen cells were lysed with 0.1 M ammonium chloride buffer. Splenocytes were cultured in triplicate (106 cells/well) in 96-well microtiter flat-bottom plates and stimulated with either purified VV previously inactivated by UV light at 1 μg/ml, purified β-Gal at 1 μg/ml (Sigma), or concanavalin A (1 μg/ml) (Sigma). Plates were incubated for 3 days at 37°C in 5% CO2. After this incubation period, proliferation assays were carried out by labeling the cells with [3H]thymidine (1 μCi/well) for 18 h. Following automated harvesting, [3H]thymidine incorporation was measured by liquid scintillation counting. Cytokine (IL-10 and gamma interferon [IFN-γ]) levels in culture supernatants were determined after 72 h of incubation. Supernatants from triplicate cultures were pooled and stored at −70°C until the assay was performed.
Evaluation of cytokine levels by ELISA.
Cytokine levels in culture supernatants and clarified spleen homogenates in PBS containing protease inhibitors (soybean trypsin inhibitor [100 μg/ml], leupeptin [10 μg/ml], and 1 mM phenylmethylsulfonyl fluoride) were determined by ELISA using the appropriate combination of antibodies from Genzyme Diagnostics (IFN-γ, IL-10, and IL-12) or PharMingen (TNF-α and IL-6). Briefly, 96-well flat-bottom plates were coated with 100 μl of anticytokine antibodies diluted in the buffer specified by the manufacturer and incubated overnight at 4°C. The wells were then washed with PBS-T and coated with PBS containing 1% BSA at 37°C for 2 h. Serial twofold dilutions of supernatants or sera and adequate dilutions of standard cytokines were added in duplicate and incubated at 37°C for 1 to 2 h. The wells were then washed with PBS-T and incubated with the specific biotinylated anticytokine antibody diluted in PBS-T with 1% BSA for 1 to 2 h. After three to four washes, wells were incubated with horseradish peroxidase-conjugated streptavidin for 15 min at 37°C and developed with tetramethylbenzidine reagent (Sigma); the reaction was stopped with 2 N H2SO4, and the absorbance was measured at 450 nm.
Evaluation of CD8+ T cells by the ELISPOT assay.
The enzyme-linked immunospot (ELISPOT) assay to detect antigen-specific CD8+ T cells was performed as previously described (20). Briefly, 96-well nitrocellulose plates were coated with 8 μg of anti-mouse IFN-γ monoclonal antibody R4-6A2 (PharMingen) per ml in 75 μl of PBS. After overnight incubation at room temperature, wells were washed three times with RPMI 1640, 100 μl of complete medium supplemented with 10% FCS was added to each well, and the plates were incubated at 37°C for 1 h. Spleen cells (depleted of erythrocytes) from different groups of mice were added in triplicate in twofold dilutions. P815 cells (a mastocytoma cell line which expresses only major histocompatibility complex class I molecules) were used as antigen-presenting cells. When the number of specific CD8+ T cells against VV antigens was evaluated, P815 cells (107 cells/ml) were infected at a multiplicity of infection (MOI) of 5 PFU of WRluc virus per cell; at 4.5 h postinfection, cells were washed and treated with mitomycin C (30 μg/ml; Sigma). When the number of CD8+ IFN-γ-secreting cells specific for the β-Gal protein was evaluated, splenocytes were restimulated in vitro for 5 to 6 days in the presence of 10−5 M synthetic peptide TPHPARIGL and 25 U of IL-2 per ml as described previously (36). The ELISPOT assay was then performed by pulsing the P815 cells with 10−6 M specific peptide and treating them with mitomycin C as described above. After several washes with culture medium, 105 P815 cells were added to each well. As control, P815 cells not pulsed with the peptide or uninfected but treated under similar conditions were used. Plates were incubated for 24 h in a 37°C incubator with a 5% CO2 atmosphere, washed extensively with PBS-T, and incubated for 2 h at 37°C with a solution of 2 μg of biotinylated anti-mouse IFN-γ monoclonal antibody XMG1.2 (PharMingen) per ml in PBS-T. Thereafter, plates were washed with PBS-T, 100 μl of peroxidase-labeled avidin (Sigma) at a 1/800 dilution in PBS-T was added to each well, and the plates were incubated at room temperature for 1 h. Wells were washed with PBS-T and PBS, and the spots were developed by adding 1 μg of the substrate DAB (Sigma) in 50 mM Tris-HCl (pH 7.5) containing 0.015% hydrogen peroxide. Spots were then counted with the aid of a Leica MZ122 APO stereomicroscope and QWIN imaging system software from Leica.
RESULTS
Differential levels of expression of recombinant genes from the attenuated MVA and wild-type WR strains of VV in permissive and nonpermissive cell lines.
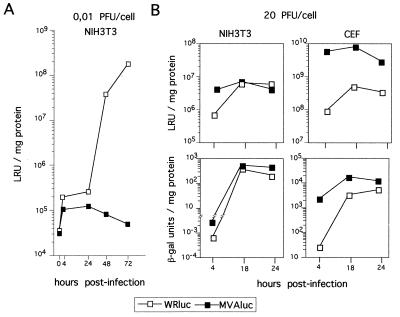
To study the replication of MVA virus in cultured cells and in animals, we constructed an rMVA virus expressing the luciferase reporter gene under control of the VV early/late P7.5 promoter (MVAluc), which allowed virus gene expression to be monitored with high sensitivity (43). This vector also encodes the reporter lacZ gene under the control of the strong VV late P11 promoter for easy selection (see Materials and Methods). Previous studies with cells in culture infected with rMVA have shown that levels of gene expression in human-derived cell lines are equal to or even higher than levels for the replication-competent WR strain (5, 53, 58, 59). Since our interest was to evaluate the in vivo immunogenicity of rMVA in mice, we have extended those previous studies to the mouse-derived cell line NIH 3T3 by measuring the expression levels of luciferase or β-Gal reporter genes after infection at low or high MOI. As a control, infection of fully permissive CEF and BHK-21 cultures was also monitored, and values were compared with those from cells infected with rWR virus expressing both reporter genes (WRluc). Infection of CEF or BHK-21 cultures at low MOI (0.01 PFU/cell) showed no major differences in the kinetics of luciferase activity between MVAluc and WRluc viruses (data not shown). However, in the mouse cell line NIH 3T3, MVAluc was unable to replicate productively, as no increase of luciferase activity with time was observed (Fig. 1A). To assess the ability of MVA to express virus-encoded proteins in NIH 3T3 cells, we infected the cells at a high MOI (20 PFU/cell). As shown in Fig. 1B, luciferase levels were nearly sixfold higher at early times (4 hpi) in MVAluc-infected cultures than in WRluc-infected cells, although the two viruses reached similar levels at 18 hpi. Late expression in those cultures measured by β-Gal production showed the same differences at early times postinfection, while at 24 hpi nearly three times more LacZ expression was present in cultures infected with MVAluc. Similar results were obtained when CEFs were infected, although differences in the level of expression of luciferase were greater (100-fold) in permissive cells than in NIH 3T3 cells at early times postinfection. Again, at 24 hpi nearly threefold more β-Gal activity was found in MVAluc-infected cultures than in cells infected with WRluc. As previously described for the murine L929 cell line (54), no signs of infection were visible with MVAluc in murine cells. Moreover, even in the permissive CEF cultures at 24 hpi, moderate cytopathic effect (CPE) appeared during MVAluc infection, whereas total cell destruction was observed following WRluc infection (data not shown). Thus, higher levels of expression from an early promoter (controlling luciferase expression) or a late promoter (driving β-Gal expression) were obtained during MVA infection than WR infection in nonpermissive cells and especially in permissive cells.
FIG. 1.
rMVA gene expression in permissive and nonpermissive cells. (A) Kinetics of VV expression after infection of murine NIH 3T3 cultures with 0.01 PFU of either MVAluc or WRluc per cell. Luciferase gene expression was monitored as an indicator of virus multiplication in cells and expressed as luciferase reference units (LRU) per milligram of protein. (B) Kinetics of VV expression after infection of CEF or NIH 3T3 cell cultures with 20 PFU of either MVAluc or WRluc per cell. At each indicated time point, cells were scraped and lysed, and luciferase and β-Gal activities in clarified supernatants were measured. β-Gal units were determined according to standards used in the assay and the manufacturer's instructions.
MVA gene expression in different mouse target tissues.
It has been reported that MVA is avirulent in both adult and newborn mouse models (34) and in irradiated mice (56). However, persistence and levels of antigen expression after in vivo administration of MVA have not been studied in this animal model. Therefore, we characterized in mice the dissemination of the avirulent MVA and WR viruses to determine the effects of deletions in the MVA genome on the ability of the virus to infect mouse tissues different from the site of inoculation. We also defined the effect that the viral dose has on the ability of MVA to reach target tissues, as well as the level of expression of foreign MVA-encoded reporter genes.
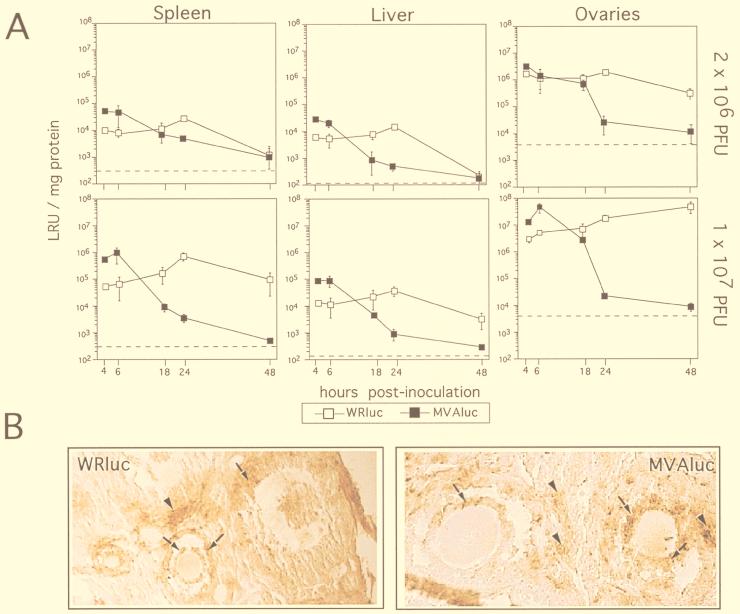
Groups of 6-week-old mice were inoculated i.p. with different doses (2 × 106 or 1 × 107 PFU/animal) of MVAluc or WRluc virus, and at different times postinoculation luciferase activity (as an index of virus replication) was determined in extracts of liver, spleen, and ovaries, using three mice per time point (Fig. 2A). After i.p. administration, MVAluc reached these organs as efficiently as the WRluc virus, as no delay in viral expression in both cases was observed at 4 hpi. Interestingly, at early-late times postinoculation (4 and 6 h), when a round of virus replication should not yet have occurred, luciferase levels found in the three organs from MVAluc-infected animals were nearly 5- to 10-fold higher than those found in tissues from WRluc-infected mice, except in the ovaries of mice given 2 × 106 PFU, where the levels were identical. In mice inoculated with MVAluc, luciferase activity decays with time, falling to background values at 48 hpi, in clear correlation with an impairment in the ability to productively replicate in the mouse tissues, as no infectious virus was detected (data not shown). Luciferase levels from MVAluc were not detected after 48 hpi, regardless of the dose of virus inoculated or the organ analyzed. In WRluc-infected tissues, luciferase activity peaked at 24 hpi to levels 10-fold higher than those at early time points, although the levels were identical or slightly lower in spleen and liver compared with those achieved at earlier times postinfection with MVAluc-infected mice. At later times postinfection, levels of luciferase were 2 and 4 log units higher for WRluc than for MVAluc.
FIG. 2.
rMVA gene expression in different target mouse tissues. (A) BALB/c mice were inoculated i.p. with 2 × 106 or with 107 PFU of either WRluc or MVAluc per animal, as indicated. At indicated times postinoculation, the extent of virus gene expression was evaluated in spleens, livers, and ovaries by the luciferase assay as described in Materials and Methods. Luciferase activity in the different homogenate tissue samples was measured and expressed as the amount of protein present in tissue extracts (luciferase reference units [LRU] per milligram of protein). Background levels in control uninfected tissues are shown as broken lines. Results represent mean values from samples of three animals per day and group with standard deviation. Similar results were obtained in two independent experiments. (B) Immunohistological staining of ovary sections taken at 18 hpi from mice infected with 107 PFU of WRluc or MVAluc. Sections were reacted with an anti-VV polyclonal serum and developed with DAB as described in Materials and Methods. Arrows indicate positive infected follicular cells; arrowheads indicate interstitial positive cells. Magnification, ×250.
To further characterize the expression of MVA proteins in target tissues, we performed immunohistochemical staining of sections from livers, spleens, and ovaries of either WRluc- or MVAluc-inoculated mice at early times postinoculation (6 and 18 hpi). The anti-VV sera used indicated that the infected regions in the ovaries from animals inoculated with either WR or MVA virus were the same, and the extents of staining, indicative of the invasiveness of each virus, were nearly identical. Areas of the ovaries positive for anti-VV sera were the follicular cells of follicles at different stages of differentiation (Fig. 2B), cells at the corpus luteum, interstitial cells, and cells in the germinal epithelium (not shown). Accessory fat cells were also stained, and scattered cells of the columnar epithelium of the uterus appeared positive for VV antigens. Only a few positive cells per slice were present in liver and spleen sections, reflecting the great differences in luciferase activity measured in those tissues compared to the ovaries.
The results presented in Fig. 2 revealed that MVAluc can infect different VV target tissues following i.p. inoculation, reaching the tissues at the same time as the WR virus and expressing viral genes at early times more efficiently than the wild-type virus. At a dose of 107 PFU, the levels of protein expression at early times after inoculation with MVAluc were nearly 10-fold higher in target tissues than in replication-competent WRluc virus-infected organs.
Characterization of the humoral immune response elicited in mice after MVA or WR infection. (i) Antibodies against VV antigens and a recombinant antigen.
Our next aim was to compare the humoral immune responses induced with the two recombinant viruses by measuring antibodies against the vector (VV antigens) and against the recombinant antigen (β-Gal). As the particle/PFU ratio could be of importance in these studies, we analyzed protein content in purified viral stocks of WRluc and MVAluc by Western blotting with polyclonal anti-VV antigens. No significant differences were observed in the total amount of protein (data not shown), indicating that comparable particle numbers were present in the two virus stocks.
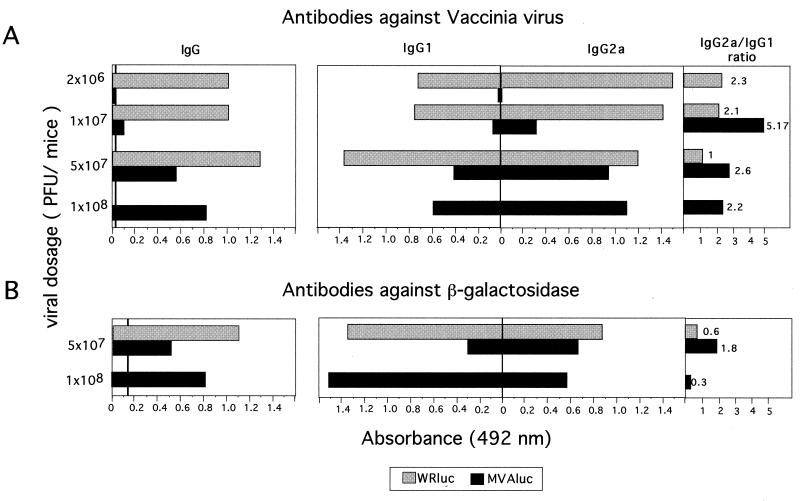
Different groups of four mice each were inoculated i.p. with graded doses of MVAluc or WRluc virus (2 × 106, 1 × 107, 5 × 107, and 1 × 108 PFU/mouse); 14 days later, total IgG and IgG subclasses against VV antigens (envelope proteins) and β-Gal in serum were measured. Infection with 108 PFU of WRluc was excluded due to mortality of mice. Serum samples from mice inoculated with the WRluc virus showed comparable and high anti-VV IgG antibodies regardless of the virus dose administered (Fig. 3A, left). However, in serum samples from mice inoculated with MVAluc, antibody levels against VV proteins were significantly lower than in serum from mice inoculated with WRluc. The ratio of Th1 to Th2 responses induced against VV antigens, measured indirectly by the IgG2a/IgG1 ratio (Fig. 3A, right), decreased when high doses of either virus were administered, indicating that the immune response was biased toward a Th2 type. However, sera from mice inoculated with MVA showed a greater Th1/Th2 ratio than sera from mice infected with WRluc, suggesting that the initial dose of MVA had less effect since MVA does not replicate in vivo postinoculation.
FIG. 3.
Humoral immune response elicited against VV antigens and the recombinant β-Gal antigen following immunization of mice with WRluc or MVAluc. Groups of four mice were inoculated i.p. with the indicated doses of virus vectors; 14 days later, blood was obtained and sera were tested for specific IgG, IgG1, and IgG2a antibodies. Absorbance values (measured at 492 nm) of specific anti-VV antibodies and β-Gal antibodies correspond to serum dilutions of 1/400 and 1/50, respectively. Values represent mean absorbances of triplicates of pooled serum samples.
Both viruses induced a significant humoral immune response against β-Gal at a dose equal to or higher than 5 × 107 PFU (Fig. 3B, left). At low doses (2 × 106 and 1 × 107 PFU), the two vectors induced similar levels of antibodies against β-Gal, barely above those in samples from naive mice (data not shown). When a dose of 5 × 107 PFU of WRluc or MVAluc was used, the differences in the level of specific antibodies against β-Gal antigen induced by the two vectors were comparable to those observed against VV antigens. For the recombinant antigen analyzed (β-Gal), the IgG1 response (Th2) was more pronounced than with VV antigens. The quantity and quality of the humoral immune response observed with a dose of 108 PFU of MVA were similar to those seen with 5 × 107 PFU of the WRluc virus.
The results in Fig. 3 indicate that the extent of the humoral immune response induced with WR and MVA viruses is dependent on the viral dose administered; only high doses of MVA elicited a significant anti-VV antigen humoral response. There was also a polarization of the immune response toward a Th2 type with increased doses of both viruses, although a more pronounced Th1 type was elicited by MVA at any dose. However, higher doses of MVAluc were required to generate an antibody response against β-Gal comparable to that elicited by WRluc.
(ii) Neutralization assays.
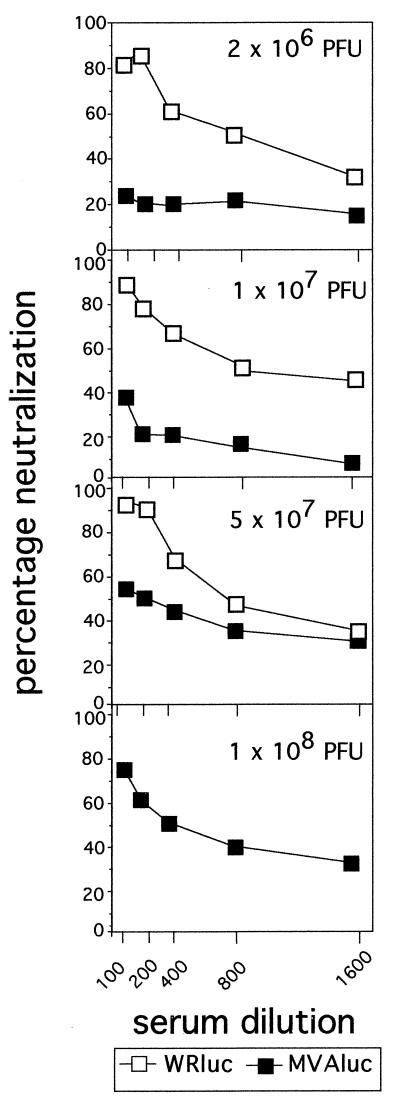
To further compare the humoral immune responses triggered by MVA and WR viruses, we analyzed the levels of neutralizing antibodies against VV by measuring the inhibition of plaque formation of wild-type WR virus on BSC-40 cells. Figure 4 shows the neutralization curves obtained with pooled sera from animals inoculated with different doses of the two rVVs. Similar neutralization titers (reciprocal of the maximum dilution that gives 50% reduction in plaque number [NT50]) were found in sera from mice inoculated with WRluc regardless of the dose administered (NT50, 800). However, in sera from mice inoculated with MVAluc, significant levels of neutralizing antibodies were present only at a dose of 5 × 107 or 1 × 108 PFU (NT50, 200 or 400, respectively); at lower doses, no significant neutralizing antibodies were detected, in clear correspondence with the levels of anti-VV IgG antibodies (Fig. 3A).
FIG. 4.
Induction of neutralizing antibodies against VV after immunization of mice with WRluc and MVAluc viruses. Fourteen days after BALB/c mice were immunized with different doses of rMVA and rWR virus (Fig. 3), neutralizing antibodies were determined. Different dilutions of sera were incubated for 1 h at 37°C with 200 PFU of wild-type WR virus, and then the inhibition of PFU formation on BSC-40 cells was measured. The percentage of neutralization was calculated as (number of PFU with immune serum/number of PFU with naive serum) × 100 obtained with pooled sera from four mice per group.
Characterization of the cellular immune response elicited after MVA or WR infection of mice. (i) CD8+ IFN-γ-secreting T cells against VV antigens.
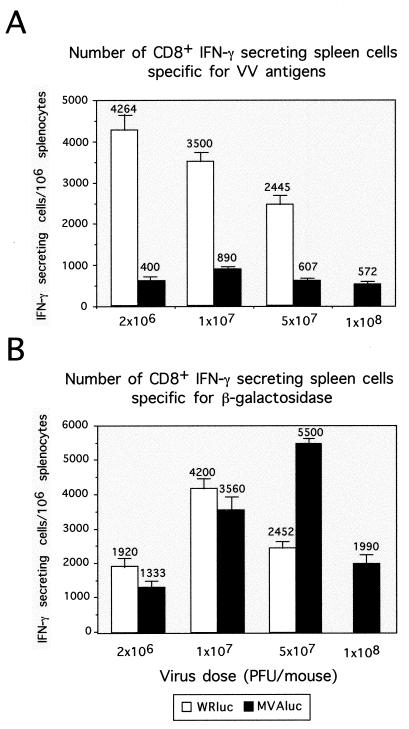
Recent studies in which MVA and WR recombinants expressing the HIV-1 envelope protein were used to compare the cellular immune response induced by the two vectors revealed that MVA may be at least as effective as the replicating WR strain in inducing a specific cytotoxic T-lymphocyte (CTL) response against the HIV antigen (6). To analyze this observation in more detail, our next approach was to carry out a comparative study of the cellular immune responses induced against VV antigens and β-Gal when MVA and WR vectors were used. Groups of four mice were inoculated i.p. with rMVA or rWR using the doses employed in the experiments described above. Ten days later, the CD8+ T-cell immune responses elicited against VV antigens and β-Gal were analyzed by the ELISPOT assay, which quantifies the number of specific major histocompatibility complex class I-restricted IFN-γ-secreting cells (Fig. 5A). In WRluc-inoculated mice, the number of CD8+ IFN-γ-secreting anti-VV T cells decreased inversely with the viral immunizing dose, being 4,264 ± 378 at a dose of 2 × 106 PFU/mice, compared with 2,445 ± 219 in mice inoculated with 5 × 107 PFU. However, animals inoculated with MVAluc showed a markedly lower CD8+ T-cell immune response against the vector at any viral dose compared to that observed upon WRluc administration. The greatest difference was observed at the lower dose (2 × 106 PFU), in which case 10 times fewer anti-VV CD8+ T cells (400 ± 72) were found in MVA-inoculated mice, whereas at doses of 107 PFU (890 ± 151) and at 5 × 107 PFU (634 ± 76), the number of anti-VV T cells was 4 times lower in the animals that received the MVA vector.
FIG. 5.
Cellular immune response elicited against VV antigens and the recombinant β-Gal antigen after immunization with different doses of rMVA or rWR virus. (A) Determination by ELISPOT assay of the number of IFN-γ-secreting CD8+ T cells specific for VV antigens. Groups of four mice were immunized i.p. with the indicated doses of MVAluc or WRluc; 10 days later, spleen cells were used as responder cells in the ELISPOT assay with P815 cells infected with WR as targets. Bars represent the average of three pooled samples ± standard deviation from triplicate cultures. Data are representative of at least two independent experiments. (B) Number of IFN-γ-secreting CD8+ T cells specific for the β-Gal peptide TPHPARIGL. Splenocytes from the different mouse groups were cultured in vitro with the β-Gal peptide (10−5 M) for 5 to 7 days. The number of specific IFN-γ-secreting CD8+ T cells was determined after coculture of restimulated splenocytes with P815 cells coated with the peptide by ELISPOT assay. Bars represent the mean ± standard deviation for triplicate cultures.
In sharp contrast with the result obtained for VV antigens, the cellular immune response against β-Gal in MVAluc-inoculated mice was equivalent to or higher than that in WRluc-inoculated mice (Fig. 5B). Animals inoculated with rWR exhibited the peak CD8+ T-cell response against β-Gal at a dose of 107 PFU (4,200 ± 282); when MVA was used, the greatest response (5,500 ± 141) appeared at the dose of 5 × 107 PFU, decreasing at 108 PFU (2,200 ± 113), in agreement with the Th1/Th2 ratios measured (Fig. 3B). The specific cellular immune response against β-Gal was determined after 5 days of specific in vitro restimulation; therefore, we cannot exclude the possibility that after this expansion period, differences in the initial number of specific T cells could be minimized if maximum cell growth levels are reached.
(ii) T-cell proliferation against VV antigens and against the recombinant antigen β-Gal.
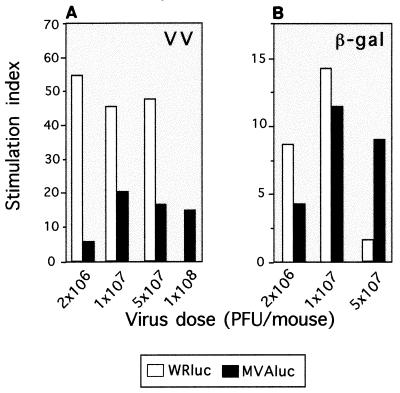
To further investigate the cellular immune response induced by both vectors against the virus and the recombinant antigen, we performed Th cell proliferation assays. Ten days after immunization, splenocytes from mice of the experiments described above were restimulated in vitro with soluble VV antigens or the recombinant β-Gal protein. When T-cell proliferation responses against VV antigens were analyzed in splenocytes from WRluc-immunized mice, a higher stimulation index (SI) was seen in those mice immunized with the lower virus dose (Fig. 6A), in agreement with the higher numbers of CD8+ IFN-γ-secreting T cells observed in the ELISPOT assay. At 1 × 107 and 5 × 107 PFU, the SI decreased slightly and was similar for both virus doses. Again, when cellular immunity against VV was evaluated in MVAluc-inoculated mice, the T-cell proliferative response was lower than the response in splenocytes from WRluc-immunized mice. The major difference in the SI against VV antigens between WRluc- and MVAluc-inoculated mice was seen at the dose of 2 × 106 PFU/mouse: the difference observed was 10-fold, similar to that for the CD8+ T-cell response. When the T-cell proliferative response against β-Gal protein was studied, differences in the magnitude of the response elicited by the two viral vectors were minor (Fig. 6B). At 107 PFU/mouse, the SI against β-Gal was comparable in mice inoculated with MVA or WR virus. However, at 5 × 107 PFU/mouse, T-cell proliferation against the recombinant protein was greater in splenocytes from MVA-inoculated mice, in agreement with the CD8+ T-cell response detected in the ELISPOT assay.
FIG. 6.
Specific Th cell proliferation after immunization of mice with WRluc or MVAluc virus. Splenocytes from mice of the same groups as used for the experiment depicted in Fig. 5 were tested for T-cell proliferation by stimulation in vitro with WR antigen (1 μg/ml; A) or β-Gal (1 μg/ml; B). After 72 h of culture, [3H]thymidine (1 μCi/well) was added; 18 h later, cells were harvested and radioactivity was measured. Bars represent the specific proliferative response measured as SI (cpm in the presence of the specific antigen/cpm in negative controls).
(iii) Pattern of cytokine secretion after VV antigen restimulation.
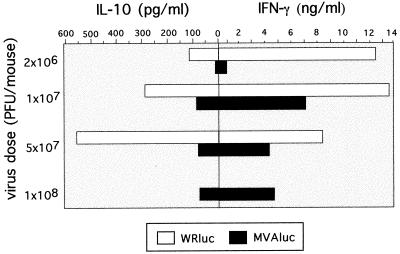
Next we examined the possible influence of the vector and virus dose on the type of Th cell immune response elicited by analyzing the pattern of cytokines expressed by T cells after in vitro restimulation with UV-inactivated VV antigen. As shown in Fig. 7, splenocytes from mice inoculated with the low dose (2 × 106 PFU) of WRluc elicited a Th1-dominant pattern of cytokines (high levels of IFN-γ and low levels of IL-10), whereas at this dose in MVAluc-inoculated mice the levels were not significant. At 107 PFU, the levels of IFN-γ secreted by splenocytes from WRluc-inoculated mice were maintained, while IL-10 levels increased. The fact that in this group of mice the immune response was biased toward the Th2 type correlated with the lower specific CD8+ T-cell response observed in the ELISPOT assay (Fig. 5A). At an MVA dose of 107 PFU/mouse, the pattern of cytokines secreted against VV antigens corresponded to a Th1-dominant type. At higher doses of MVA (5 × 107 and 1 × 108 PFU), the levels of IFN-γ decreased (Fig. 7) in correlation with the lower number of anti-VV CD8+ IFN-γ-secreting T cells (Fig. 5).
FIG. 7.
Th1 and Th2 cytokine secretion pattern after immunization of mice with WRluc or MVAluc. Levels of IFN-γ and IL-10 in supernatants of lymphocyte cultures from mice inoculated with MVAluc or WRluc were measured after 72 h of restimulation in vitro in the presence of VV antigens (1 μg/ml). Averages from triplicate cultures are shown.
Characterization of the cellular immune response elicited upon MVA or WR inoculation showed that the CMI induced against the vector was stronger in those mice that received the WR recombinant that in those animals inoculated with the attenuated MVA vector. Nevertheless, the MVA vector was capable of inducing a similar or even greater (at a dose of 5 × 107 PFU/mouse) specific cellular immune response against the β-Gal antigen, as evaluated by CD8+ T-cell ELISPOT and Th cell in vitro restimulation assays.
Differences in profiles of early proinflammatory cytokines in mice inoculated with rMVA or rWR.
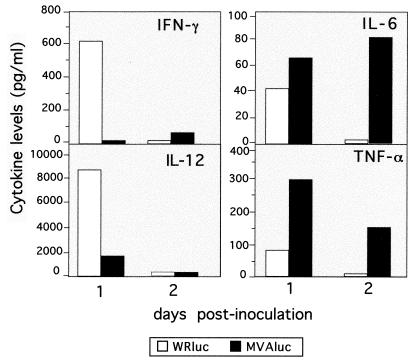
It has been recently reported (8) that MVA, in contrast to the WR strain, does not express soluble receptor analogues that bind IFN-γ and IFN-α/β, which may explain the good immunogenicity of MVA in spite of its poor replication. To determine whether MVA and WR induced different early inflammatory responses that could affect the further specific immune response elicited, we analyzed the pattern of cytokines produced in the spleen at early times after inoculation with rMVAluc or rWRluc. For this purpose, we inoculated i.p. each of four mice with a dose of 5 × 107 PFU of MVAluc or WRluc and at 1 and 2 days postinoculation (dpi) evaluated levels of IFN-γ, IL-12, IL-6, and TNF-α in homogenate lysates from pooled spleen samples (Fig. 8). One day after infection, only the mice that received the WRluc virus showed levels of both IFN-γ and IL-12 significantly different from background levels found in naive control mice (Fig. 8A). In contrast, levels of IL-6 and TNF-α were 1.5- and 4-fold higher, respectively, at 1 dpi in MVA-inoculated mice than in mice receiving WR. Moreover, these differences were even greater at 2 dpi: background levels were found in mice given WR, while the levels in MVA-inoculated mice were higher (IL-6) or slightly lower (TNF-α) than at 1 dpi. These findings showed that the two viral vectors exhibited different profiles of proinflammatory cytokines early after infection, possibly reflecting differences in genetic background and the timing and levels of antigen expression.
FIG. 8.
In vivo proinflammatory cytokine secretion after immunization of mice with MVAluc or WRluc virus. Groups of eight mice were inoculated i.p. with 5 × 107 PFU of MVAluc or WRluc; 1 and 2 days later, spleens were excised and stored at −70°C. Cytokines were measured by ELISA from pooled samples of spleen homogenates prepared in PBS with protease inhibitors as described in Materials and Methods.
DISCUSSION
The development of safe live-virus-based vectors represents a challenge of crucial importance due to their proven ability to activate the immune system at both humoral and cellular levels. Poxvirus-derived vectors are promising since they were successfully used during the smallpox vaccination program (57) and also in field animal experiments. Moreover, it has been recently established that VV vectors are among the most efficient vectors during boosting irrespective of the delivery system used at priming (35). However, elimination of pathogenic characteristics must be ensured for widespread acceptance as safe vaccines, as undesirable side effects, particularly in immunosuppressed individuals, are an issue with VV vectors as anti-HIV vaccines. Moreover, due to the long-term immunity developed in smallpox vaccinees, this apparent advantage represents an added difficulty, as no strong immune response against new VV-encoded foreign antigens would be elicited in people with a history of smallpox. Indeed, clinical data support this idea, as previously reported (12). Therefore, efforts to improve VV vectors, focusing on the generation of safe VV vectors as well as the development of new vaccination strategies, are under way. MVA is being considered as a safe attenuated VV-derived vector for development of live-virus-based vaccines (37). However, despite the potential of MVA as a vaccine vector, little is known about its effects in vivo, in particular (i) ability to reach and infect target tissues other than the site of inoculation, (ii) period of MVA persistence to trigger expression of encoded genes, and (iii) immunological defenses which are raised against the vector. To define the biological properties exhibited by MVA following immunization, we have examined in the BALB/c mouse model the in vivo expression of MVA-driven reporter genes, as well as the CMI and humoral responses induced against the vector and to recombinant β-Gal expressed by the virus. We then compared the results with those obtained after inoculation of mice with the virulent rWR virus.
First, we analyzed virus gene expression in cultured cells, using reporter genes (luciferase and lacZ), by infecting nonpermissive mouse NIH 3T3 cells and permissive CEF cultures with either rWRluc or rMVAluc. We found the reporter gene levels higher for the MVA recombinant, regardless of the permissivity of the cells to the virus (Fig. 1). These differences were more pronounced in CEFs, reflecting the adaptation of MVA to grow in avian cells. The higher gene expression from MVA occurs in the absence of visible CPE in NIH 3T3 cell cultures, in agreement with findings previously described (8); only partial CPE was found in CEFs at 24 hpi, when total cell destruction was present in WR-infected cultures. Such enhancement of protein expression from MVA has been previously reported, but in our virus-cell system, both rWR and rMVA were generated with the same transfer vector (pSC11luc) (43), suggesting that early events in the MVA life cycle and/or advantages in transcription or translation due to multiple deletions in functional genes could account for the more efficient gene expression of MVA, which is cell type independent.
To monitor MVA infection in vivo, luciferase activity was measured in target tissues (spleen, liver, and ovaries) of mice infected with MVAluc or WRluc. We chose the i.p. route of inoculation to achieve a systemic infection that could allow the virus to reach all potential target tissues. We found that MVAluc-driven protein expression peaks earlier and lasts for a shorter period of time than during WRluc infection, reaching nearly 10-times-higher luciferase levels at early times in the spleen and liver, in agreement with in vitro data (see above). With a systemic route of virus inoculation (i.p.), luciferase activity was found in the same target tissues as upon WRluc inoculation, indicating that MVA has retained the ability to infect the same tissues as the WR strain. This finding was further corroborated by immunohistochemical labeling of infected ovaries with a hyperimmune anti-VV serum: at either 6 or 18 hpi, both the intensity of the signal and the infected regions were undistinguishable between WR- and MVA-infected ovaries, further evidence that luciferase activity is a good indicator of viral infection in vivo. The sharp fall in luciferase activity seen in MVAluc-infected tissues at 18 hpi (spleen and liver) and 24 hpi (ovaries) (Fig. 2A) suggests a limitation in viral spread from the initially infected target cells, since replication-competent WR gene expression increases until 24 hpi in the spleen and liver and later in ovaries, when luciferase activity in MVA-infected tissues was close to background levels. Despite the differences in kinetic profiles and with the exception of infection in ovaries, the maximum levels of luciferase activity were nearly identical for the two rVVs. These findings demonstrate the main differences in in vivo behavior between the MVA vector and a wild-type vector.
Preexisting immunity to viral antigens is an important consideration with respect to developing vaccines based on rVV, as immunity against the heterologous gene product can be prevented when a second dose is delivered in repeated immunizations with rVV vectors or in vaccinee against smallpox. Although rMVA and rWR can trigger similar immune responses against recombinant proteins (6, 23, 54), the antiviral immune parameter previously analyzed in vivo in comparison with other VV strains was the antibody titer against the vector (23). Here we present a more exhaustive comparative study of the immune response generated against the MVA vector, addressing both the humoral and the cellular immune responses. We found that when animals were immunized with rWR virus, levels of specific anti-VV IgGs were high and nearly identical at any of the assayed doses, whereas rMVA induced a significant humoral response at only the two higher doses used. However, in the monkey model (23), similar anti-VV antibodies were detected in animals immunized by different routes with MVA or the Wyeth VV strain, indicating that results may be specific for both the animal model and the route of inoculation used. The anti-VV CD8+ T-cell immune response determined by the ELISPOT assay revealed that at any dose, MVA elicited a CMI response 4- to 10-fold lower than that when WR was inoculated. The latter showed a clear dose dependence, as the numbers of IFN-γ-secreting CD8+ T cells decreased with increasing virus dose significantly (P < 0.01).
The extent of the immune response elicited for specific antigens depends primarily on antigen dose (30, 40), although the sustained expression of antigens appears to be even more relevant than antigen dose during the generation of a T-cell response (26). Although the levels of foreign antigen expression at early times postinfection are the same or even higher in MVA- than in WR-infected mouse tissues, the short time span in which expression occurs leads to less sustained viral gene expression. Moreover, as MVA does not multiply productively, levels of viral antigens are lower during MVA infection, which could explain the weaker humoral response elicited against VV antigens. Very large amounts of antigen often result in specific T-cell and sometimes B-cell unresponsiveness, which could explain why there is no enhancement in the anti-VV IgG levels, as well as the suppression of the CMI responses raised with increased WR dose, a phenomenon not observed upon MVA infection. Hence, viral dose and viral attenuation are factors affecting the level and duration of expression of antigen produced, and this in turn determines the final outcome of the immune response. In this regard, antibody-dependent immunological memory and memory CTL precursor frequencies have different requirements with respect to antigen persistence, as described for other viral infections (3, 4, 28).
A meaningful finding was that the lower levels of anti-VV IgG circulating antibodies in MVA-inoculated mice were also reflected in lower levels of anti-VV neutralizing antibodies. The low antibody and CMI responses and more specifically the poor neutralizing antibodies induced by MVA have important implications for repeated immunizations, as the appearance of neutralizing antibodies in sera correlates with immunity to VV (10). Thus, low neutralizing titers against the vector might lead to an increased antibody response to a foreign antigen after repeated immunizations with an rVV based on the MVA vector, facilitating multiple inoculations with poxvirus vectors. Indeed, previous data obtained with the SIV-macaque model showed an increase in the SIV-specific antibody titer after repeated rMVA injections (23) as well as in the specific CTL response (50).
The anti-β-Gal humoral immune response triggered by rVV correlated with the anti-VV response, as those mouse groups that showed higher anti-VV antibodies also had higher levels of anti-β-Gal antibodies. Similar observations have been described for other attenuated VV strains (16, 24, 29, 51), in which lower anti-VV antibodies correlated with lower levels of antibodies to specific recombinant proteins, which are directly modulated by the extent of replication of the viral vector. The specific anti-β-Gal CMI was found to be lower than that against VV antigens, as no measurable counts were obtained in fresh ELISPOT assays (data not shown). Interestingly, when the ELISPOT assay was performed after 5 days of specific in vitro stimulation, we found that despite the relatively weak anti-VV immune response elicited, MVA induced a CMI against the foreign β-Gal product equal to that induced by WR. Furthermore, it was twofold higher than in WR-infected mice at the dose that elicited the strongest response (5 × 107 PFU) (P < 0.01). It appears that the CMI against β-Gal follows a response with a peak that is achieved at higher doses for MVA than for WR inoculation and that maximum CMI levels can be obtained during infection with the MVA vector.
Concerning the skewing of the immune responses induced by the two viral vectors, determined by different methods (Fig. 3 and 6), our findings indicate a Th1-to-Th2 polarization as the viral dose increases. It is well established that both the affinity and the amount of the antigen play a major role in activating a particular arm of the immune system (38) and modulating the strength of T-cell signaling, which in turn can affect dramatically the balance of Th1 and Th2 subsets (9). It is noteworthy that at all doses assayed, the strength of the Th1 response is higher during MVA immunization, probably due to the low levels of circulating viral antigens, as no mature particles are produced, whereas during WR infection, viral particles produced increase the initial viral input.
We also studied the induction of proinflammatory cytokines at early times postinfection during MVA inoculation in comparison with WR inoculation. Here we show that MVA infection induces higher splenic levels of IL-6 and TNF-α cytokines at 1 and 2 dpi than during WR infection. Our observations are in concordance with other studies in which after mucosal MVA or WR immunization, higher levels of IL-6 and TNF-α were found for monocyte cells from Peyer's patches of MVA-inoculated mice upon lipopolysaccharide stimulation (5). In contrast, levels of IFN-γ and IL-12 in the same spleen samples were higher than in samples from WR-inoculated mice, with a peak at 24 hpi. These results indicate that MVA and WR viruses induced different profiles of proinflammatory cytokines, which may be relevant in modulating the ultimate specific immune response elicited. Indeed, different viral infections induce distinct earlier cytokine responses; for example, mouse cytomegalovirus (MCMV) induces the production of IL-6, a key mediator of induction of glucocorticoids that can suppress multiple immune functions including CMI (27). Accordingly, during MCMV infection, the induced glucocorticoid response seems to be responsible for the relatively weak T-cell response (46). In our study, the lower antiviral cellular immune response induced by MVA inoculation might be explained in part by the differential IL-6 cytokine induction and its possible influence on glucocorticoid production, which may have a downstream immune effect on the antiviral response. As MVA does not express the soluble receptor for IFN-α that is encoded by WR (52), it is tempting to speculate that deletion of this gene could play a role in the inflammatory response induced. In this regard, MVA induces high levels of IFN-α/β in primary human cells (8). If it does so also in vivo, it could account for the lower IFN-γ and IL-12 levels found in MVA-infected mice in comparison with those given WR, since IFN-α/β has an inhibitory action on IL-12 and IFN-γ production in vivo and in vitro during lymphocytic choriomeningitis virus and MCMV infection (13).
In conclusion, in this study we have demonstrated the behavior of MVA in mice, in particular its ability to express antigens in target organs after i.p. inoculation and the differences in the immune responses elicited in comparison with the virulent WR strain. The most significant findings are the low antivirus immune responses elicited at both humoral and cellular levels and the low neutralization titers achieved, which might be of relevance in future vaccination schemes. However, the CMI raised against a late foreign antigen (β-Gal) was at certain viral doses higher than that triggered by a WR vector; also, antibodies to β-Gal were not very different for either virus at the optimal dose. Moreover, the patterns of proinflammatory cytokines induced early upon viral infection were different for MVA and WR: higher levels of IL-6 and TNF-α and no significant IL-12 and IFN-γ were induced after MVA inoculation, while these two cytokines were induced during WR infection. Overall, the findings presented here may be relevant to the rational design of MVA-based vaccines, as benefits derived from the low immunogenicity of the vector without affecting the immune response to foreign antigens can be applied in future vaccination protocols using VV-derived vectors.
ACKNOWLEDGMENTS
J.C.R. and M.M.G. contributed equally to this work.
We thank Antonio Alcamí and Margarita del Val for critical reading of the manuscript. We are also indebted to Dolores Rodriguez for expert advice about handling of MVA at early stages of this work. The excellent technical assistance of M. Victoria Jimenez is also acknowledged.
This work was supported by grants 08.6/0020/97 from the Comunidad Autónoma de Madrid, SAF98-0056 from the Comision Interministerial de Ciencia y Tecnología, Spain, and BIO4-CT98-0456 from the European Union. J.C.R. and M.M.G. are recipients of postdoctoral fellowships from the Comunidad Autónoma de Madrid, Spain, and Consejo Nacional de Investigaciones Científicas y Técnicas de Argentina, respectively.
REFERENCES
- 1.Altenburger W, Süter C-P, Altenburger J. Partial deletion of the human host range in the attenuated vaccinia virus MVA. Arch Virol. 1989;105:15–27. doi: 10.1007/BF01311113. [DOI] [PubMed] [Google Scholar]
- 2.Antoine G, Scheiflinger F, Dorner F, Falkner F G. The complete genomic sequence of the modified vaccinia Ankara strain: comparison with other Orthopoxviruses. Virology. 1998;244:365–396. doi: 10.1006/viro.1998.9123. [DOI] [PubMed] [Google Scholar]
- 3.Bachmann M F, Odermatt B, Hengartner H, Zinkernagel R M. Induction of long-lived germinal centers associated with persisting antigen after viral infection. J Exp Med. 1996;183:2259–2269. doi: 10.1084/jem.183.5.2259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bachmann M F, Kündig T M, Hengartner H, Zinkernagel R M. Protection against immunopathological consequences of a viral infection by activated but not resting cytotoxic T cells: T cell memory without “memory T cells”? Proc Natl Acad Sci USA. 1997;94:640–645. doi: 10.1073/pnas.94.2.640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Belyakov I M, Wyatt L S, Ahlers J D, Earl P, Pendleton C D, Kelsall B L, Strober W, Moss B, Berzofsky J A. Induction of a mucosal cytotoxic T-lymphocyte response by intrarectal immunization with a replication-deficient recombinant vaccinia virus expressing human immunodeficiency virus 89.6 envelope protein. J Virol. 1998;72:8264–8272. doi: 10.1128/jvi.72.10.8264-8272.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Belyakov I M, Moss B, Strober W, Berzofsky J A. Mucosal vaccination overcomes the barrier to recombinant vaccinia immunization caused by preexisting poxvirus immunity. Proc Natl Acad Sci USA. 1999;96:4512–4517. doi: 10.1073/pnas.96.8.4512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bender B S, Rowe C A, Taylor S F, Wyatt L S, Moss B, Small P A., Jr Oral immunization with a replication-deficient recombinant vaccinia virus protects mice against influenza. J Virol. 1996;70:6418–6424. doi: 10.1128/jvi.70.9.6418-6424.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Blanchard T J, Alcamí A, Andrea P, Smith G L. Modified vaccinia virus Ankara undergoes limited replication in human cells and lacks several immunomodulatory proteins: implications for use as a human vaccine. J Gen Virol. 1998;79:1159–1167. doi: 10.1099/0022-1317-79-5-1159. [DOI] [PubMed] [Google Scholar]
- 9.Bluestone J A. New perspectives of CD28-B7-mediated T cell costimulation. Immunity. 1995;2:555–559. doi: 10.1016/1074-7613(95)90000-4. [DOI] [PubMed] [Google Scholar]
- 10.Buller R M, Palumbo G J. Poxvirus pathogenesis. Microbiol Rev. 1991;55:80–122. doi: 10.1128/mr.55.1.80-122.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Carroll M W, Overwijk W, Chamberlain R S, Rosenberg S A, Moss B, Restifo N P. Highly attenuated modified vaccinia virus Ankara (MVA) as an effective recombinant vector: a murine tumor model. Vaccine. 1997;15:387–394. doi: 10.1016/s0264-410x(96)00195-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cooney E L, Collier A C, Greenberg P D, Coombs R W, Zarling J, Arditti D E, Hoffman M C, Hu S L, Corey L. Safety of and immunological response to a recombinant vaccinia virus vaccine expressing HIV envelope glycoprotein. Lancet. 1991;337:567–572. doi: 10.1016/0140-6736(91)91636-9. [DOI] [PubMed] [Google Scholar]
- 13.Cousens L P, Orange J S, Sue H C, Biron C A. Interferon-α/β inhibition of interleukin 12 and interferon-γ production in vitro and endogenously during viral infection. Proc Natl Acad Sci USA. 1997;94:634–639. doi: 10.1073/pnas.94.2.634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cox W I, Tartaglia J, Paoletti E. Poxvirus recombinants as live vaccines. In: Binns M M, Smith G L, editors. Recombinant poxviruses. Boca Raton, Fla: CRC Press; 1992. pp. 123–162. [Google Scholar]
- 15.Dallo S, Esteban M. Isolation and characterization of attenuated mutants of vaccinia virus. Virology. 1987;159:408–422. doi: 10.1016/0042-6822(87)90480-6. [DOI] [PubMed] [Google Scholar]
- 16.Dallo S, Maa J-S, Rodriguez J R, Rodriguez D, Esteban M. Humoral immune response elicited by highly attenuated variants of vaccinia virus and by an attenuated recombinant expressing HIV-1 envelope protein. Virology. 1989;173:323–329. doi: 10.1016/0042-6822(89)90250-x. [DOI] [PubMed] [Google Scholar]
- 17.Drexler I, Heller K, Wahren B, Erfle V, Sutter G. Highly attenuated modified vaccinia virus Ankara replicates in baby hamster kidney cells, a potential host for virus propagation, but not in various human transformed and primary cells. J Gen Virol. 1998;79:347–352. doi: 10.1099/0022-1317-79-2-347. [DOI] [PubMed] [Google Scholar]
- 18.Durbin A P, Wyatt L S, Siew J, Moss B, Murphy B R. The immunogenicity and efficacy of intranasally or parenterally administered replication-deficient vaccinia-parainfluenza virus type 3 recombinants in rhesus monkeys. Vaccine. 1998;16:1324–1330. doi: 10.1016/s0264-410x(98)00010-3. [DOI] [PubMed] [Google Scholar]
- 19.Fenner F. Risks and benefits of vaccinia vaccine use in the worldwide smallpox eradication campaign. Res Virol. 1989;140:465–466. doi: 10.1016/s0923-2516(89)80126-8. [DOI] [PubMed] [Google Scholar]
- 20.Gherardi M M, Ramirez J C, Rodriguez D, Rodriguez J R, Sano G, Zavala F, Esteban M. IL-12 delivery from recombinant vaccinia virus attenuates the vector and enhances the cellular immune response against HIV-1 env in a dose-dependent manner. J Immunol. 1999;162:6724–6733. [PubMed] [Google Scholar]
- 21.Hanke T, Blanchard T J, Schneider J, Hannan C M, Becker M, Gilbert S C, Hill A V S, Smith G L, McMichael A. Enhancement of MHC class I-restricted peptide-specific T cell induction by a DNA prime/MVA vaccination regime. Vaccine. 1998;16:439–445. doi: 10.1016/s0264-410x(97)00226-0. [DOI] [PubMed] [Google Scholar]
- 22.Hanke T, Blanchard T J, Schneider J, Ogg G S, Tan R, Becker M, Gilbert S C, Hill A V S, Smith G L, McMichael A. Immunogenicities of intravenous and intramuscular administrations of modified vaccinia virus Ankara-based multi-CTL epitope vaccine for human immunodeficiency virus type 1 in mice. J Gen Virol. 1998;79:83–90. doi: 10.1099/0022-1317-79-1-83. [DOI] [PubMed] [Google Scholar]
- 23.Hirsch V, Fuerst T R, Sutter G, Carroll M W, Yang L C, Golstein S, Piatak M, Elkins W R, Alvord W G, Montefiori D, Moss B, Lifson J D. Patterns of viral replication correlate with outcome in simian immunodeficiency virus (SIV)-infected macaques: effect of prior immunization with a trivalent SIV vaccine in modified vaccinia virus Ankara. J Virol. 1996;70:3741–3752. doi: 10.1128/jvi.70.6.3741-3752.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Holzer G W, Remp G, Antoine G, Pfleiderer M, Enzersberger O M, Emsenhuber W, Hämmerle T, Gruber F, Urban C, Falkner F G, Dorner F. Highly efficient induction of protective immunity by a vaccinia virus vector defective in late gene expression. J Virol. 1999;73:4536–4542. doi: 10.1128/jvi.73.6.4536-4542.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jin X, Bauer D E, Tuttleton S E, Lewin S, Gettie A, Blanchard J, Irwin C E, Safrit J T, Mittler J, Weinberg L, Kostrikis L G, Zhang L, Perelson A S, Ho D D. Dramatic rise in plasma viremia after CD8+ T cell depletion in simian immunodeficiency virus-infected macaques. J Exp Med. 1999;189:991–998. doi: 10.1084/jem.189.6.991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kündig T M, Shahinian A, Kawai K, Mittrücker H-W, Sebzda E, Bachmann M F, Mak T W, Ohashi P S. Duration of TCR stimulation determines costimulatory requirement of T cells. Immunity. 1996;5:41–52. doi: 10.1016/s1074-7613(00)80308-8. [DOI] [PubMed] [Google Scholar]
- 27.Kunicka J E, Talle M A, Denhart G H, Brown M, Prince C A, Goldstein G. Immunosuppression by glucocorticoids: inhibition of production of multiple lymphokines by in vivo administration of dexamethasone. Cell Immunol. 1993;149:39–49. doi: 10.1006/cimm.1993.1134. [DOI] [PubMed] [Google Scholar]
- 28.Lau L L, Jamieson B D, Somasundaram T, Ahmed R. Cytotoxic T-cell memory without antigen. Nature. 1994;369:648–652. [PubMed] [Google Scholar]
- 29.Lee M S, Roos J M, McGuigan L C, Smith K A, Cornier N, Cohen L K, Roberts B E, Payne L G. Molecular attenuation of vaccinia virus: mutant generation and animal characterization. J Virol. 1992;66:2617–2630. doi: 10.1128/jvi.66.5.2617-2630.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Liew F Y, Parish C R. Lack of correlation between cell-mediated immunity to the carrier and the carrier-hapten helper effect. J Exp Med. 1974;139:779–784. doi: 10.1084/jem.139.3.779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mackett M, Smith G L, Moss B. Vaccinia virus: a selectable eukaryotic cloning and expression vector. Proc Natl Acad Sci USA. 1982;79:7415–7419. doi: 10.1073/pnas.79.23.7415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mayr A, Hoschstein-Mintzel V, Stickl H. Abstammung, eigenschaftenund Verwendung des attenuierten Vaccinia-Stammes MVA. Infection. 1975;3:6–16. [Google Scholar]
- 33.Mayr A, Stickl H, Muller H K, Denner K, Singer H. The smallpox vaccination strain MVA: marker, genetic structure, experience gained with the parenteral vaccination and behaviour in organisms with a debilitated defence mechanism. Zentbl Backteriol Hyg B. 1978;167:375–390. [PubMed] [Google Scholar]
- 34.Meyer H, Sutter G, Mayr A. Mapping of deletions in the genome of highly attenuated vaccinia virus MVA and their influence on virulence. J Gen Virol. 1991;72:1031–1038. doi: 10.1099/0022-1317-72-5-1031. [DOI] [PubMed] [Google Scholar]
- 35.Miyahira Y, García-Sastre A, Rodriguez D, Rodriguez J R, Murata K, Tsuji M, Palese P, Esteban M, Zavala F, Nussenzweig R S. Recombinant viruses expressing a human malaria antigen elicit protective immune CD88+ T cell responses in mice. Proc Natl Acad Sci USA. 1998;95:3954–3959. doi: 10.1073/pnas.95.7.3954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Montoya M, del Val M. Intracellular rate-limiting steps in MHC class I antigen processing. J Immunol. 1999;163:1914–1922. [PubMed] [Google Scholar]
- 37.Moss B, Carroll M W, Wyatt L S, Bennink J R, Hirsch V, Golstein S, Elkins W R, Fuerst T R, Lifson J D, Piatak M, Restifo N P, Overwijk W, Chamberlain R, Rosenberg S A, Sutter G. Host range restricted non-replicating vaccinia virus vectors as vaccine candidates. Adv Exp Med Biol. 1996;397:7–13. doi: 10.1007/978-1-4899-1382-1_2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Murray J S. How the MHC selects Th1/Th2 immunity? Immunol Today. 1998;19:157–163. doi: 10.1016/s0167-5699(97)01237-1. [DOI] [PubMed] [Google Scholar]
- 39.Panicali D, Paoletti E. Construction of poxvirus as cloning vectors: insertion of the thymidine kinase gene from herpes simplex virus into the DNA of infectious vaccinia virus. Proc Natl Acad Sci USA. 1982;79:4927–4931. doi: 10.1073/pnas.79.16.4927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Parish C R. Immune deviation: a historical perspective. Immunol Cell Biol. 1996;74:449–456. doi: 10.1038/icb.1996.75. [DOI] [PubMed] [Google Scholar]
- 41.Redfield R R, Wright D C, James W D, Jones T S, Brown C, Burke D S. Disseminated vaccinia in a military recruit with human immunodeficiency virus (HIV) disease. N Engl J Med. 1987;316:673–676. doi: 10.1056/NEJM198703123161106. [DOI] [PubMed] [Google Scholar]
- 42.Rodríguez D, Rodríguez J R, Rodríguez J F, Trauber D, Esteban M. Highly attenuated vaccinia virus mutants for the generation of safe recombinants viruses. Proc Natl Acad Sci USA. 1989;86:1287–1291. doi: 10.1073/pnas.86.4.1287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rodríguez J F, Rodríguez D, Rodríguez J R, McGowan E B, Esteban M. Expression of the firefly luciferase gene in vaccinia virus: a highly sensitive gene marker to follow virus dissemination in tissues of infected animals. Proc Natl Acad Sci USA. 1988;85:1667–1671. doi: 10.1073/pnas.85.5.1667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rodríguez J R, Risco C, Carrascosa J L, Esteban M, Rodríguez D. Characterization of early stages in vaccinia virus membrane biogenesis: implications of the 21-kilodalton protein and a newly identified 15-kilodalton envelope protein. J Virol. 1997;71:1821–1833. doi: 10.1128/jvi.71.3.1821-1833.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rowland-Jones S, Tan R, McMichael A J. The role of cellular immunity in protection against HIV infection. Adv Immunol. 1997;65:277–346. [PubMed] [Google Scholar]
- 46.Ruzek M C, Millert A H, Opal S M, Pearce B D, Biron C A. Characterization of early cytokine responses and an interleukin (IL-6)-dependent pathway of endogenous glucocorticoid induction during murine cytomegalovirus infection. J Exp Med. 1997;185:1185–1192. doi: 10.1084/jem.185.7.1185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Scheleimer R P, Jacques A, Shin H S, Lichstein L M, Plaut M. Inhibition of T cell-mediated cytotoxicity by anti-inflammatory steroids. J Immunol. 1984;132:266–271. [PubMed] [Google Scholar]
- 48.Schmitz J E, Kuroda M J, Santra S, Sasseville V G, Simon M A, Lifton M A, Racz P, Tenner-Racz K, Dalesandro M, Scallon B J, Ghrayeb J, Forman M A, Montefiori D C, Rieber E P, Letvin N L, Reimann K A. Control of viremia in simian immunodeficiency virus infection by CD8+ lymphocytes. Science. 1999;283:857–860. doi: 10.1126/science.283.5403.857. [DOI] [PubMed] [Google Scholar]
- 49.Sedegah M, Jones T R, Kaur M, Hedstrom R, Hobart P, Tine J A, Hoffman S L. Boosting with recombinant vaccinia increases immunogenicity and protective efficacy of malaria DNA vaccine. Proc Natl Acad Sci USA. 1998;95:7648–7653. doi: 10.1073/pnas.95.13.7648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Seth A, Ourmanov I, Kuroda M J, Schmitz J E, Carroll M W, Wyatt L S, Moss B, Forman M A, Hirsch V M, Letvin N L. Recombinant modified vaccinia virus Ankara-simian immunodeficiency virus gag pol elicits cytotoxic T lymphocytes in rhesus monkeys detected by a major histocompatibility complex class I/peptide tetramer. Proc Natl Acad Sci USA. 1998;95:10112–10116. doi: 10.1073/pnas.95.17.10112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Shida H, Hinuma Y, Hatanaka M, Morita M, Kidokoro M, Suzuki K, Maruyama T, Takahashi-Nishimaki F, Sugimoto M, Kitamura R, Miyazawa T, Hayami M. Effects and virulences of recombinant vaccinia viruses derived from attenuated strains that express the human T-cell leukemia virus type I envelope gene. J Virol. 1988;62:4474–4480. doi: 10.1128/jvi.62.12.4474-4480.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Smith G L, Symons J A, Khanna A, Vanderplasschen A, Alcamí A. Vaccinia virus immune evasion. Immunol Rev. 1997;159:137–154. doi: 10.1111/j.1600-065x.1997.tb01012.x. [DOI] [PubMed] [Google Scholar]
- 53.Sutter G, Moss B. Nonreplicating vaccinia vector efficiently expresses recombinant genes. Proc Natl Acad Sci USA. 1992;89:10847–10851. doi: 10.1073/pnas.89.22.10847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sutter G, Wyatt L S, Foley P L, Bennink J R, Moss B. A recombinant vector derived from the host-range-restricted and highly attenuated MVA strain of vaccinia virus stimulates protective immunity in mice to influenza virus. Vaccine. 1994;12:1032–1040. doi: 10.1016/0264-410x(94)90341-7. [DOI] [PubMed] [Google Scholar]
- 55.Taylor J, Weinberg R, Tartaglia J, Richardson C, Alkhatib G, Breidis D, Appel M, Norton E, Paoletti E. Nonreplicating viral vectors as potential vaccines: recombinant canarypox virus expressing measles virus fusion (F) and hemagglutinin (HA) glycoproteins. Virology. 1992;187:321–328. doi: 10.1016/0042-6822(92)90321-f. [DOI] [PubMed] [Google Scholar]
- 56.Werner G T, Jentsch U, Metzger E, Simon J. Studies on poxvirus infection in irradiated animals. Arch Virol. 1980;64:247–256. doi: 10.1007/BF01322704. [DOI] [PubMed] [Google Scholar]
- 57.World Health Organization. The global eradication of smallpox. Final report of the global commission for the certification of smallpox eradication. History of international public health, no. 4. Geneva, Switzerland: World Health Organization; 1980. [Google Scholar]
- 58.Wyatt L, Moss B, Rozenblatt S. Replication-deficient vaccinia virus encoding bacteriophage T7 RNA polymerase for transient gene expression in mammalian cells. Virology. 1995;210:202–205. doi: 10.1006/viro.1995.1332. [DOI] [PubMed] [Google Scholar]
- 59.Wyatt L, Shors S T, Murphy B R, Moss B. Development of a replication-deficient recombinant vaccinia virus vaccine effective against parainfluenza virus 3 infection in an animal model. Vaccine. 1996;14:1451–1458. doi: 10.1016/s0264-410x(96)00072-2. [DOI] [PubMed] [Google Scholar]