Hidradenitis suppurativa (HS) is a chronic inflammatory skin disease showing recurrent painful nodules, abscesses, and draining sinus tracts. Hurley clinical staging I/II/III defines disease severity. We defined the epithelial cell fate at single-cell resolution in severe late-stage II/III HS and identified genome-wide chromatin rewiring that contributes to high-resolution transcription profiles of stem/progenitor follicular epithelial cells of which HS-S100A and Basal III elevate inflammatory cytokines production (1). We also defined inflammatory-gene enhancers and their coordinated TFs in HS basal CD49fhigh cells and identified pharmacological target IRF3 (1), a cGAS-STING signaling component (2).
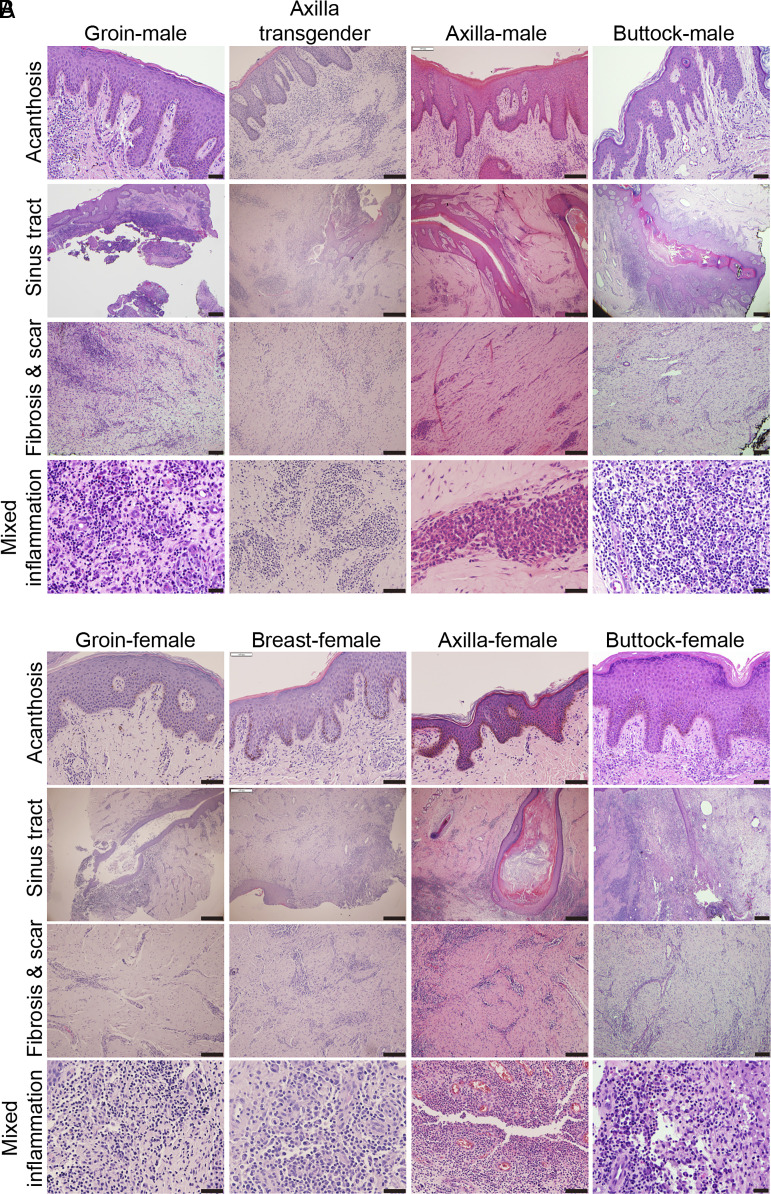
Nebo et al. (3) argue that our findings have limitations because of (1) noncomparability of the case and control skin samples, (2) differences based on the anatomical distribution of skin biopsies and sex, and (3) extensive heterogeneity in cellular composition/molecular signatures. We disagree with this representation as they inaccurately reflect the disease pathogenesis. Gender and racial differences in epidemiological data largely relate to the incidence (penetrance) of disease, rather than disease stages, treatment profile outcome, or tissue histology. Disease stage-specific variability between anatomical sites of males and females, and Caucasians and Blacks is unknown. Thus, the features of stage II/III HS skin from different anatomical locations (groin, axilla, buttock, breast, gluteal cleft, etc.) from male, transgender, and female patients manifest no significant differences in acanthosis, sinus tracts, fibrosis, scar, and inflammation (Fig. 1). Similarly, no significant differences have also been reported in cytokine profile and treatment regimen in these patient groups (4, 5).
Fig. 1.
H & E staining showing histologic features of hidradenitis suppurativa. The clinical specimens represent groin, axilla, and buttock of male or transgender patients (A) and groin, breast, axilla, and buttock (gluteal cleft) in female patients (B). (Scale bar: 100 µm.)
Inclusion of Control Human Skin
Disease-site-specific matched collection of appropriate number of normal skin samples is a herculean task. Therefore, most studies utilize disease-adjacent histologic normal skin, skin from mammaplasty/abdominoplasty, or neonatal foreskin but none of these are truely matched controls. We used control skin from mammaplasty/abdominoplasty (1).
Variation of Transcriptome among Various Anatomical Regions and Differences in the Molecular Drivers of HS
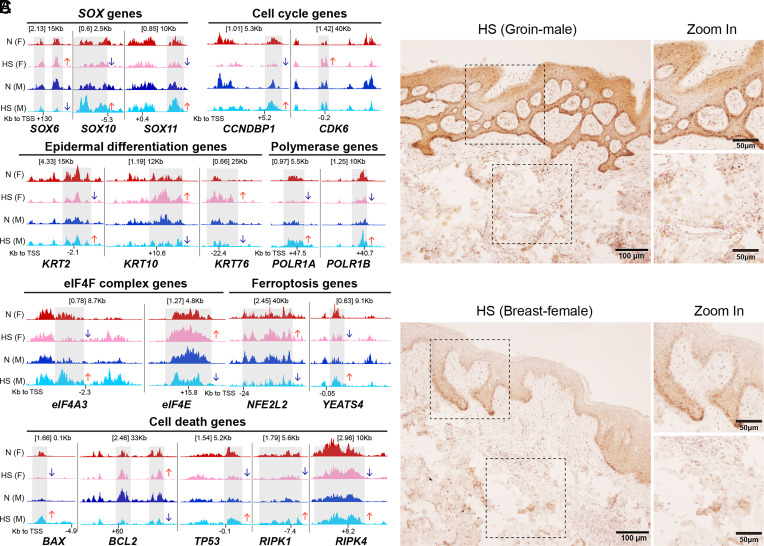
Undisputably, variation exists in the transcriptome of skin from distinct anatomical sites (6) which may alter disease penetrance. None-the-less, once HS has progressed to late stages, differences in disease-driving tissue-specific transcriptomics are not yet described. As shown in Fig. 2A, a subset of gene loci with H3K4me1 occupancy differ in HS skin from various patient cohorts (CUT&RUNseq), which are likely gender dependent as they show opposing effects in male and female. However, these data need to be further confirmed in a larger cohort of patients.
Fig. 2.
Epigenetic modifications and activation of RIP kinase 3 in HS males and females. (A) Enrichment of H3K4me1 CUT&RUN peaks on gene loci from normal and HS skin of male and female cohorts. The genes include SOX family, epidermal differentiation, cell cycle and RNA transcription & translation, and cell death/ferroptosis. Genome coverage tracks with scaled signal per million reads and shows enriched signals from the CD49f high epidermal cells. n = 1 for each gender-related skin condition. The arrow indicates the difference in HS as compared to the normal control. Gray boxes highlight the altered signaling regions. N, normal; F, female; M, male. (B and C) Immunohistochemical staining showing similar pattern for expression of phospho-RIP kinase3 in samples from various anatomical sites of HS patients. The clinical specimens represent groin of male patient (B) and breast of female patient (C). (Scale bar: 50 or 100 µm as indicated.)
Heterogeneity in Cellular Composition and Molecular Signatures in Stage II/III HS
Although heterogeneity in the cellular and molecular composition in HS across various anatomic sites of the normal skin is well-known (7), distinct disease-driving populations with a unique molecular signature are currently unfounded. We show by immunochemistry staining that expression of activated RIPK3, a key kinase involved in tissue disruption/remodeling by necroptosis (8), is similar in HS skin from different anatomical areas (Fig. 2B).
Androgen Signaling (AS)
The AS mainly in female patients is evident from the increased perimenstrual HS flares and benefit from antiandrogenic drugs (spironolactone, finasteride, metformin, etc.) (9, 10). However, the underlying molecular mechanism remains poorly understood. In murine models, AS is known to dampen cutaneous inflammation in males (11) but the same remains unclear in humans, particularly in HS immunopathogenesis.
Acknowledgments
Author contributions
L.J., C.R., and M.A. designed research; L.J., M.P.K., and S.T.H. performed research; L.J., M.P.K., C.L., and M.A. analyzed data; and L.J., C.R., and M.A. wrote the paper.
Competing interests
The authors declare no competing interest.
Contributor Information
Lin Jin, Email: ljin@uabmc.edu.
Mohammad Athar, Email: mohammadathar@uabmc.edu.
References
- 1.Jin L., et al. , Epigenetic switch reshapes epithelial progenitor cell signatures and drives inflammatory pathogenesis in hidradenitis suppurativa. Proc. Natl. Acad. Sci. U.S.A. 120, e2315096120 (2023), 10.1073/pnas.2315096120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Orvain C., et al. , Hair follicle stem cell replication stress drives IFI16/STING-dependent inflammation in hidradenitis suppurativa. J. Clin. Invest. 130, 3777–3790 (2020), 10.1172/JCI131180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nebo I., Frew J. W., Gudjonsson J. E., Petukhova L., Tissue comparability and bias in hidrandenitis suppurativa transcriptomic studies. Proc. Natl. Acad. Sci. U.S.A. 121, e2404503121 (2024). [DOI] [PubMed] [Google Scholar]
- 4.Navrazhina K., et al. , Epithelialized tunnels are a source of inflammation in hidradenitis suppurativa. J. Allergy Clin. Immunol. 147, 2213–2224 (2021), 10.1016/j.jaci.2020.12.651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zouboulis C. C., et al. , Secukinumab in patients with moderate to severe hidradenitis suppurativa based on prior biological exposure: An efficacy and safety analysis from SUNSHINE and SUNRISE phase III trials. Br. J. Dermatol. 1–10 (2024), 10.1093/bjd/ljae098. [DOI] [PubMed] [Google Scholar]
- 6.Yan Y., et al. , Transcriptomic heterogeneity of skin across different anatomic sites. J. Invest. Dermatol. 143, 398–407.e395 (2023), 10.1016/j.jid.2022.08.053. [DOI] [PubMed] [Google Scholar]
- 7.Wiedemann J., et al. , Differential cell composition and split epidermal differentiation in human palm, sole, and hip skin. Cell Rep. 42, 111994 (2023), 10.1016/j.celrep.2023.111994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ai Y., Meng Y., Yan B., Zhou Q., Wang X., The biochemical pathways of apoptotic, necroptotic, pyroptotic, and ferroptotic cell death. Mol. Cell 84, 170–179 (2024), 10.1016/j.molcel.2023.11.040. [DOI] [PubMed] [Google Scholar]
- 9.Abu Rached N., et al. , The role of hormones in hidradenitis suppurativa: A systematic review. Int. J. Mol. Sci. 23, 15250 (2022), 10.3390/ijms232315250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Quinlan C., Kirby B., Hughes R., Spironolactone therapy for hidradenitis suppurativa. Clin. Exp. Dermatol. 45, 464–465 (2020), 10.1111/ced.14119. [DOI] [PubMed] [Google Scholar]
- 11.Hoffmann J. P., Liu J. A., Seddu K., Klein S. L., Sex hormone signaling and regulation of immune function. Immunity 56, 2472–2491 (2023), 10.1016/j.immuni.2023.10.008. [DOI] [PubMed] [Google Scholar]