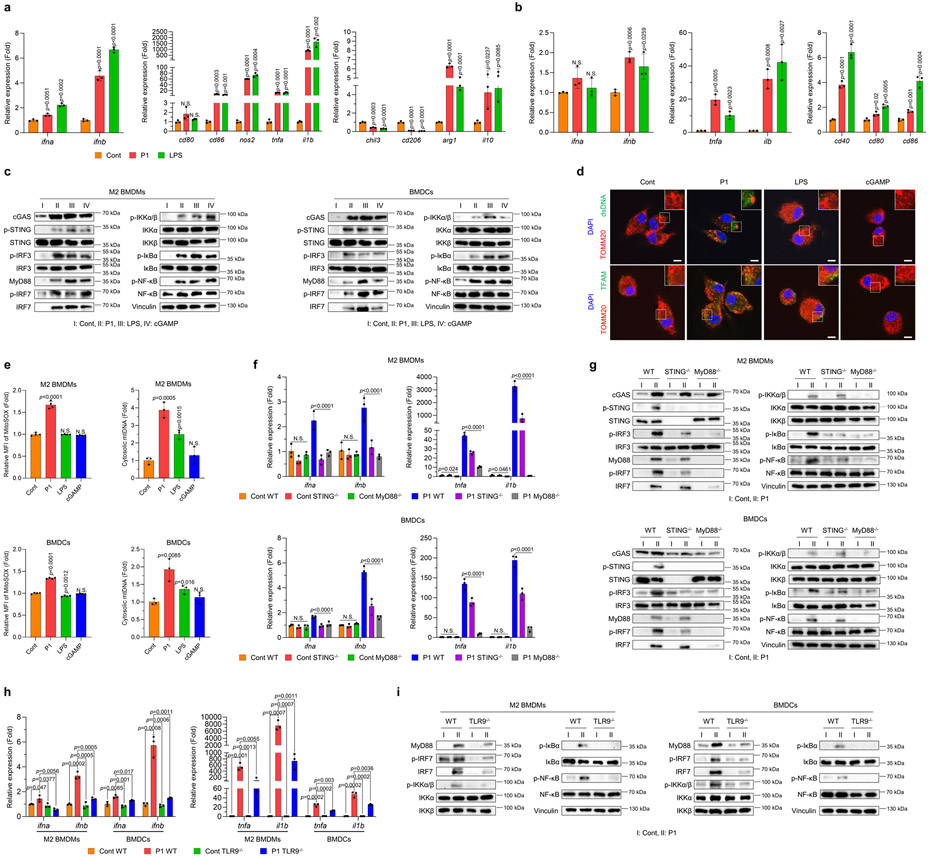
Fig. 3. P1 activates innate immune sensors by releasing mtDNA.
(a) P1 upregulated the expression of type I interferon (IFN)-related and M1-associated genes, but reduced that of M2-related genes, in M2 macrophages, as determined by quantitative reverse transcription polymerase chain reaction (RT-qPCR) (n=3, mean±SD); unpaired Student’s t test in comparison to control (Cont). (b) P1 treatment of BMDCs increased the expression of genes for type I IFNs, pro-inflammatory cytokines and co-stimulatory molecules on DC surfaces (n=3, mean±SD). (c) Western blots show that P1 stimulated cGAS-STING, MyD88, and NF-κB pathways in M2 BMDMs and BMDCs. (d) P1 triggered release of transcription factor A, mitochondrial (TFAM) and double-stranded DNA (dsDNA) from mitochondria in M2 macrophages, as visualized by confocal laser scanning microscopy (scale bar 7.5 μm). (e) Treatment of M2 BMDMs and BMDCs with P1 led to increased levels of mitochondrial ROS, as evaluated by flow cytometry (n=4, mean±SD) and promoted mtDNA release, as quantified by RT-qPCR (n=3, mean±SD); unpaired Student’s t test in comparison to Cont. (f) P1 treatment led to increased expression of genes encoding type I IFNs and pro-inflammatory cytokines in wild-type APCs, but not in STING−/− or MyD88−/− ones (n=3, mean±SD); Ordinary one-way ANOVA. (g) Western blots of proteins related to cGAS-STING, MyD88, and canonical NF-κB pathways in STING−/− or MyD88−/− APCs. (h) TLR9 deficiency in M2 macrophages and BMDCs treated with P1 reduced the expression of genes encoding type I IFNs and pro-inflammatory cytokines (n=3, mean±SD); unpaired Student’s t test in comparison to Cont. (i) TLR9-depleted APCs downregulated the MyD88-IRF7 and canonical NF-κB axes.