Figure 4.
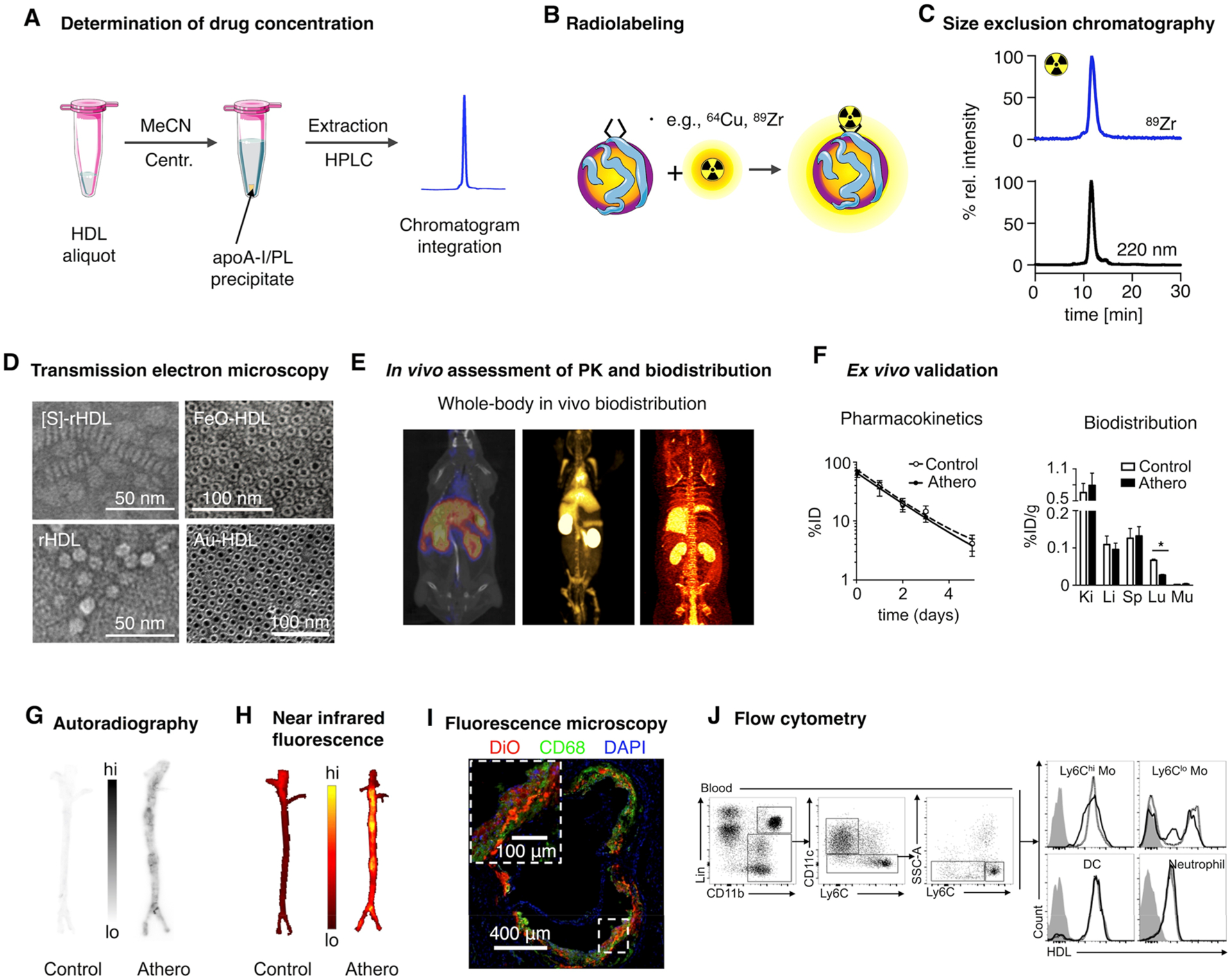
Characterization of HDL nanobiologics. (A) HDL drug content can be determined by HPLC. (B) Radiolabeling of HDL nanobiologics. (C) Size exclusion chromatography can be used to determine particle size and to confirm successful labeling of HDL and radiochemical purity. (D) Definitive size and morphology characterization should be performed by transmission electron microscopy. Reproduced with permission from refs 5, 11, and 14. Copyright 2013 and 2008 American Chemical Society, and 2014 Nature Publishing Group, respectively. (E) HDL labeling with positron emitters like 89Zr allows quantitative assessment of pharmacokinetics and biodistribution. (F) Ex vivo gamma counting and (G) autoradiography. Adapted with permission from ref 8. Copyright 2016 American College of Cardiology. (H) Evaluation of tissue distribution of HDL nanobiologics by NIRF imaging and cellular specificity by (I) fluorescence microscopy and (J) flow cytometry. Reproduced with permission from refs 14 and 8. Copyright 2014 Nature Publishing Group and 2016 American College of Cardiology, respectively.
