Figure 6.
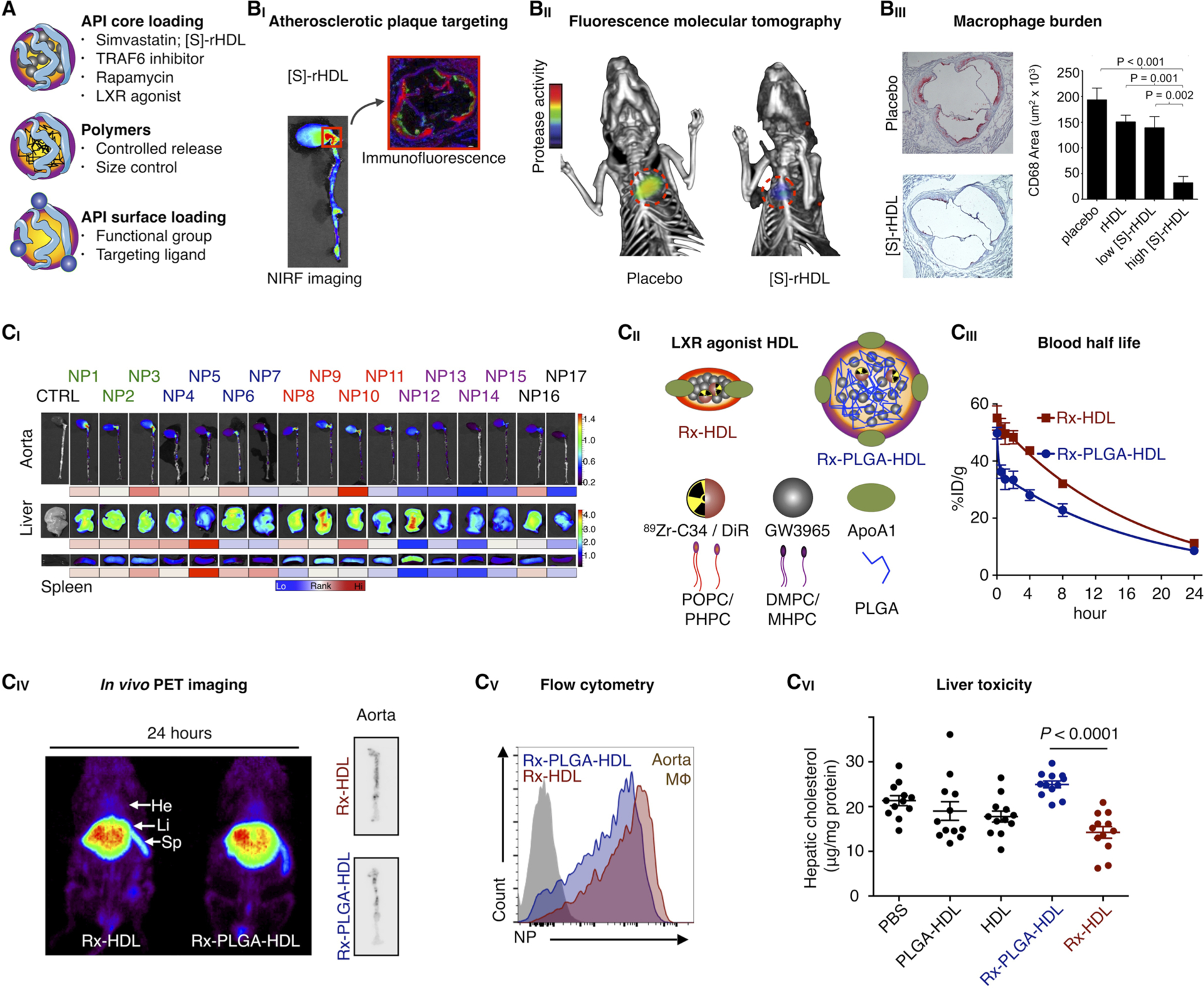
Immunotherapy with HDL nanobiologics. (A) HDL nanobiologics suitable for immunotherapy can include therapeutically active payloads or controlled release polymers in the core or compounds can be integrated in the corona. (BI) Simvastatin-loaded HDL nanobiologics ([S]-rHDL) accumulate in atherosclerotic plaques in mice, as observed by NIRF imaging of excised aortas and immunofluorescence. (BII) A [S]-rHDL treatment regimen consisting of 4 intravenous doses in 1 week, drastically reduces vessel wall inflammation in atherosclerotic mice, as quantified by FMT/CT imaging, (BIII) corresponding with a > 80% macrophage burden reduction. Reproduced with permission from ref 14. Copyright 2014 Nature Publishing Group. (CI) The HDL nanobiologic library presented in Figure 3C was screened in atherosclerotic mice. Accumulation in the aorta and target organs at 24 h post-intravenous administration is shown. In addition, the library’s pharmacokinetics and immune cell specificity was investigated. (CII) An LXR agonist was incorporated in two selected HDL nanobiologics, with very diverse in vivo properties, rendering Rx-HDL and Rx-PLGA-HDL. (CIII) Blood half-lives, (CIV) biodistribution, atherosclerotic plaque targeting, and (CV) plaque macrophage specificity were established from 89Zr-labeled Rx-HDL and Rx-PLGA-HDL. (CVI) The Rx-PLGA-HDL nanobiologic, which accumulates in the liver more pronouncedly, generated liver toxicity, while for the Rx-HDL nanobiologic liver toxicity was abolished. Reproduced with permission from ref 15. Copyright 2016 National Academy of Sciences of the United States of America.
