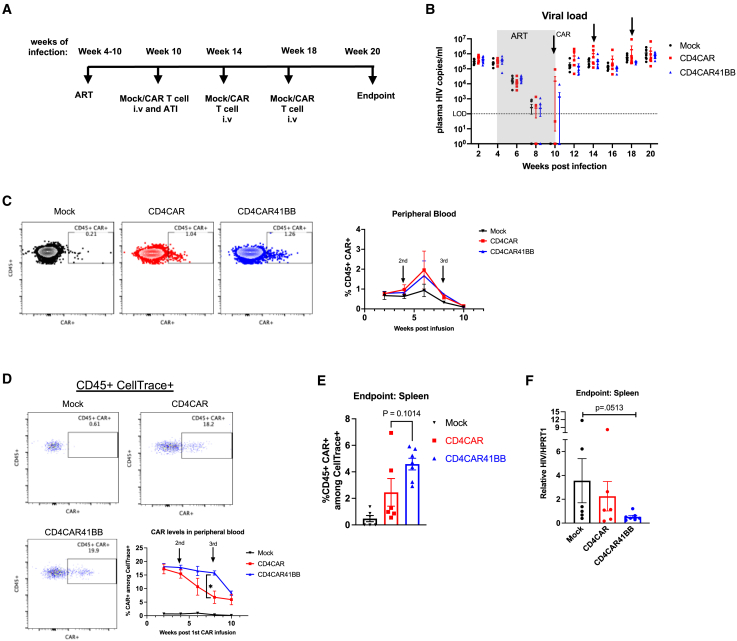
Figure 3.
CD4CAR 41BB T cells do not reduce or delay plasma viral rebound but lower tissue viral burden
(A) Experimental design: humanized TKO BLT mice were infected with 200 ng HIVNFNSXSL9 for 4 weeks, and then treated with ART for 6 weeks. At week 6 of ART, mice received 10 million mock, CD4CAR, or CD4CAR41BB transduced T cells followed 2 days later by ATI. Two additional rounds of mock or CAR T cell infusion were administered at 14 and 18 weeks postinfection. (B) Average plasma HIV RNA copies per milliliter in mock- or CAR-treated mice measured by RT-PCR. Dotted line indicates limit of detection. Arrows indicate mock or CAR treatment. (C) Representative FACS plots showing CAR+ (EGFR+) among human CD45 gating (left) and summary of percentage of CAR+ (EGFR+) cells in the human CD45+ population in peripheral blood (right). (D) Representative FACS plots showing CD45+ CAR+ (EGFR+) among CellTrace+ population gating and summary of percentage of CD45+ CAR+ cells within the CellTrace+ population in peripheral blood. (E) Percentage of CD45+ CAR+ cells within the CellTrace+ population in spleen at the endpoint of the study. (F) Relative HIV RNA levels in spleen at the endpoint of the study measured by RT-PCR. (C)–(E) measured by flow cytometry. ∗p < 0.05; significance determined 2-way ANOVA (D) and Mann-Whitney test (E and F). Data represent n = 6–7 mice per group. Error bars represent SEM.