Abstract
Objectives
We determined the clinical characteristics and prevalence of metabolic syndrome among adult Filipinos with overt hypothyroidism.
Methodology
This is a cross-sectional study of 151 adults. Patients were recruited by sequential enrollment. Anthropometric and blood pressure measurements were performed followed by blood extraction for metabolic parameters and thyroid function tests. Clinical and laboratory characteristics were compared between patients with and without metabolic syndrome.
Results
The prevalence of metabolic syndrome is 40.4% (95%CI: 32.5%, 48.7%). Patients with metabolic syndrome have a waist circumference of 88.4 ± 7.7 cm in females and 93.3 ± 9.0 cm in males. The median fasting blood glucose was 111.4 (52.2) mg/dL, median systolic blood pressure of 120 (30) mm Hg and diastolic blood pressure of 80 (20) mmHg, median serum triglycerides of 174.3 (114.2) mg/dL, median HDL-C of 42.3 (19.2) mg/dL and a proportion of patients with diabetes (23.0%) and hypertension (44.3%), respectively. The presence of increased waist circumference is the most prevalent component seen among hypothyroid patients. There were no differences in terms of age, sex, etiology of hypothyroidism and anti-TPO levels in those with and without metabolic syndrome.
Conclusion
The prevalence of metabolic syndrome in adult Filipinos with hypothyroidism is high. Emphasis must be placed on early screening using waist circumference and metabolic parameters among hypothyroid patients who are at high risk of developing metabolic syndrome
Keywords: dyslipidemia, hypothyroidism, metabolic syndrome, prevalence
INTRODUCTION
The presence of thyroid disease is associated with metabolic abnormalities due to the effects of thyroid hormones on nearly all major metabolic pathways.1 Thyroid hormones are responsible for the regulation of basal energy expenditure through their effects on catabolism.2 One of the most common metabolic abnormalities in overt hypothyroidism is dyslipidemia characterized by the increase in total and low-density lipoprotein cholesterol (LDL-C) levels and less often high-density lipoprotein cholesterol (HDL-C), serum triglycerides (TG), lipoprotein (a), apolipoprotein A1 and apolipoprotein B levels.3-6 A similar pattern has also been seen among patients with subclinical hypothyroidism.4-6
Of note, the total and LDL-C significantly improve after thyroxine replacement treatment in both forms of hypothyroidism. The dyslipidemia seen in hypothyroidism often coexists with other metabolic abnormalities including hypertension, insulin resistance and oxidative stress, all of them being risk factors for cardiovascular disease.7-11
Metabolic syndrome (MS) is a clustering of cardiometabolic risk factors that increase an individual’s risk for atherosclerotic cardiovascular disease (ASCVD), stroke and diabetes mellitus (DM). The threshold criteria vary among definitions suggested by organizations such as the International Diabetes Federation (IDF), the National Cholesterol Education Programme Adult Treatment Panel III (NCEP/ATP III) or the World Health Organization.12 It is defined as the presence of at least 3 of the following features: (1) visceral obesity depending on race; (2) raised triglyceride level or on specific treatment; (3) reduced HDL cholesterol or on specific treatment; (4) raised blood pressure or on treatment of previously diagnosed hypertension; and (5) raised fasting plasma glucose, on drug treatment or previously diagnosed type 2 diabetes based on the IDF criteria.
Worldwide, the prevalence of MS in the general population ranges from 12.5% to 31.4% depending on the definition used.13 In the Philippines, the prevalence of MS in the general population ranges from 11.9% to 51.0% depending on the criteria used.12,14,15 Likewise, the prevalence of MS in hypothyroidism varies depending on the population: 36.19% in a recent retrospective cross-sectional local study in the Philippine General Hospital, Manila, Philippines,16 40.0% in Nigeria,17 53.24% in Southern India18 and 51.8% in Venezuela19. In general, the prevalence of MS is higher among those with hypothyroidism compared to thyrotoxicosis.17-20 These findings are supported by a study in Nepal looking at the prevalence of thyroid dysfunction among 169 adults diagnosed with MS by the NCEP/ATP III criteria where 26.6% have subclinical hypothyroidism, 3.5% have overt hypothyroidism, in contrast to only 1.7% having subclinical hyperthyroidism.20 In the Philippines, the Philippine Thyroid Diseases Study (PhilTiDeS 1) showed that thyroid dysfunction was more common among women. The national prevalence of thyroid function abnormalities is 8.5%, true hypothyroidism and subclinical hypothyroidism have the prevalence rates of 0.4% and 2.2%, respectively.21 However, there is still a paucity of local data and knowledge gaps regarding the prevalence of MS in patients with thyroid dysfunction22, especially among Asians where we develop diabetes and cardiovascular diseases at much lower BMI.23,24 Hence, in this study, we determined the clinical characteristics and prevalence of MS among adult Filipino patients with hypothyroidism seen in our outpatient thyroid clinic.
METHODOLOGY
Patients and procedures
A sequential enrollment of 151 patients diagnosed with newly diagnosed overt hypothyroidism was conducted in our outpatient thyroid clinic at the Philippine General Hospital. A minimum sample size of 86 patients was determined based on the formula
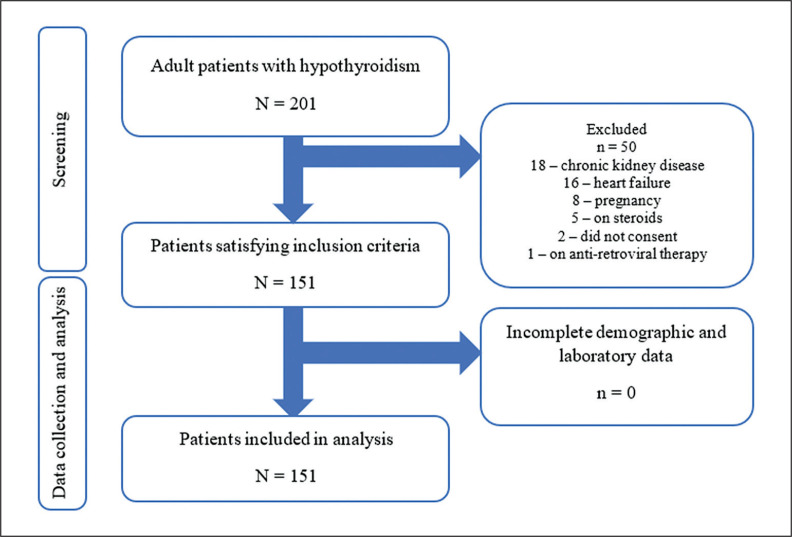
where n is the sample size, Z is 1.96, the statistic corresponding to 95 % confidence (α of 0.05), P, 6.0% (past local study) is the prevalence and e, 0.05 is the assumed error estimate. We sequentially recruited patients starting from January 1 to December 31, 2021, during their outpatient consultation using the inclusion criteria: All adult patients who are 19 years old and above diagnosed with either (1) autoimmune hypothyroidism; (2) post-procedural hypothyroidism; (3) subclinical hypothyroidism; (4) central hypothyroidism; and (5) any of the aforementioned patients from 1 – 4 on glucose-lowering therapy or cholesterol-lowering drugs. All patients with the following: patients with active/untreated thyroid malignancies, amiodarone and drug-induced hyper- or hypothyroidism (except radioactive iodine) and all patients with any of the following comorbidities: chronic liver disease, kidney disease, congestive heart failure, pregnancy, patients on oral contraceptive pills, cancer chemotherapy and anti-retroviral therapy were excluded from the study. A summary of the patient recruitment flowchart is shown in Figure 1.
Figure 1.
Flowchart of patient recruitment process.
Following informed consent, a trained research assistant obtained demographic data (age, sex, comorbidities – hypertension, diabetes mellitus, myocardial infarction, and stroke) using our approved data collection forms from patient interviews, patient’s electronic medical records and physical chart entries. The same trained research assistant was assigned to measure anthropometric measurements [weight, height, body mass index (BMI), and waist circumference] and blood pressure. The anthropometric measurements were measured as follows: the patient’s weight (rounded to the nearest tenth) and height (rounded to the nearest ones) were measured using the Detecto adult physician weighing and height scale, calibrated using known weights of 50 and 250 lbs, respectively; waist circumference (rounded to the nearest tenth) was measured using a tape measure in centimeters by passing the instrument through the umbilicus and using the bilateral iliac crests as landmarks; body mass index was calculated by dividing the patient’s weight in kilograms by the patient’s height in meters squared. Auscultatory blood pressure (rounded to the nearest ones) was measured in a seated patient using a calibrated desk-type Baxtel sphygmomanometer calibrated against an Omron digital sphygmomanometer after every 6 months of use. We used the first Korotkoff sound to mark the systolic blood pressure and the last Korotkoff sound as the diastolic blood pressure. All measurements were conducted 3 times and the average measurement was recorded in the data collection forms. This was then followed by venipuncture of 10 milliliters of blood. Blood collection for thyroid function tests and serum chemistry was performed prior to the initiation of thyroid hormone replacement. Five milliliters of blood contained in a red top tube was sent to the radioisotope laboratory for the measurement of the following: A. thyroid stimulating hormone (TSH), B. free thyroxine (FT4), and C. anti-thyroid peroxidase (anti-TPO) antibodies using the radioimmunoassay method (Beckman Coulter) while another 5 milliliters of blood contained in a red top specimen tube was sent to the medical research laboratory for the measurement of A. fasting blood glucose (FBS), B. total cholesterol (TC), C. high-density lipoprotein cholesterol (HDL-C), D. low-density lipoprotein cholesterol (LDL-C) and E. triglycerides (TG) using the colorimetric enzyme assay method. All blood samples were screened by our laboratory technician for adequacy prior to running. All laboratory personnel were blinded to the patient’s clinical data.
Statistical analysis
All data collected were encoded, tabulated and summarized using Microsoft Office Excel 2016. Data analysis was performed using Stata version 17.0 with a p-value of less than 0.05 considered significant for all tests.
Clinical and laboratory characteristics of the hypothyroid patients were summarized by descriptive statistics. Numerical variables were described as mean and standard deviations (SD), if the data was normally distributed as assessed by the Shapiro-Wilk test for normality and as median and IQR, if otherwise. Categorical variables were described as counts and proportions. BMI was categorized using the WHO Asia Pacific classification while diagnosis of MS was determined using the IDF criteria.12 The prevalence estimate of MS among adult patients with hypothyroidism was computed using a 95% confidence interval as the total number of patients satisfying the IDF criteria for diagnosis12 divided by the total number of patients enrolled. The different clinical and laboratory characteristics were compared between the 2 groups: with MS and without MS. Significant differences in the mean waist circumference between the 2 groups were determined by two-way ANOVA to adjust for the blocking variable sex. Differences in means, medians or mean ranks between the 2 groups were determined by the Student t-test or Mann-Whitney U test, as appropriate. The heterogeneity of the proportions of the different categorical variables between the 2 groups was determined by the chi-square test. Thyroid function tests and thyroid autoantibody levels were also compared in the same manner. In addition, these thyroid tests were also compared according to the number of MS components that the patients have. The thyroid function tests and metabolic parameters were also compared among those with MS according to the etiology. One-way ANOVA was used for the normally distributed variables, while the Kruskal-Wallis test was used for otherwise. Post-hoc analysis by multiple comparisons using the Dunn method was used to identify the specific etiology that has significantly different TSH, FT4 and metabolic parameters.
Ethics
The study was approved by the Research Ethics Board of the University of the Philippines Manila (UPMREB CODE: 2020-605-01) prior to commencement and was conducted in accordance with the Declaration of Helsinki. All study participants provided written informed consent prior to any study-related procedures.
RESULTS
The baseline characteristics of our patients are summarized in Table 1. The male-to-female ratio was 1:2 (52:99 patients). There were no significant differences in terms of age. Primary hypothyroidism comprised 90.1% (n = 136) of the cohort; post-procedural causes comprised 76.2% (n = 115) and autoimmune causes at 13.9% (n = 21). Our results showed that the overall prevalence of MS among patients with hypothyroidism is 40.4% (95%CI: 32.5%, 48.7%). Among males, the prevalence is 40.4% (95%CI: 30.7%, 50.7%) while it is 40.4% (95%CI: 27.0%, 54.9%) among females.
Table 1.
Clinical characteristics of patients stratified based on age, sex, waist circumference, BMI, co-morbidities and etiology of hypothyroidism
| Characteristics | Overall (n = 151) | (+) Metabolic syndrome (n = 61) | (-) Metabolic syndrome (n = 90) | p |
|---|---|---|---|---|
|
Age (years), n (%) 18 - 40 41 - 60 ≥60 |
55 (36.4%) 70 (46.4%) 26 (17.2%) |
14 (23.0%) 33 (54.1%) 14 (23.0%) |
41 (45.6%) 37 (41.1%) 12 (13.3%) |
0.015* |
|
Sex, n (%) Female Male |
99 (65.6%) 52 (34.4%) |
40 (65.6%) 21 (34.4%) |
59 (65.6%) 31 (34.4%) |
0.998* |
|
Waist circumference (cm), mean (sd) Among females Among males |
83.2 (10.4) 87.6 (9.6) |
88.4 (7.7) 93.3 (9.0) |
79.8 (10.5) 83.7 (7.9) |
<0.001** |
|
BMI (kg/m2), n (%) <18.5 18.5 - 22.9 23.0 - 24.9 25.0 - 29.9 ≥30.0 |
10 (6.8%) 52 (35.4%) 32 (21.8%) 40 (27.2%) 13 (8.8%) |
1 (1.7%) 13 (22.0%) 16 (27.1%) 21 (35.6%) 8 (13.6%) |
9 (10.2%) 39 (44.3%) 16 (18.2%) 19 (21.6%) 5 (5.7%) |
0.005* |
|
Comorbidities, n (%) Diabetes mellitus Hypertension Myocardial infarction Stroke |
19 (12.6%) 35 (23.2%) - - |
14 (23.0%) 27 (44.3%) - - |
5 (5.6%) 8 (8.9%) - - |
0.002* <0.001* - - |
|
Etiology, n (%) Post-procedural Autoimmune Central |
115 (76.2%) 21 (13.9%) 15 (9.9%) |
44 (72.1%) 10 (16.4%) 7 (11.5%) |
71 (78.9%) 11 (12.2%) 8 (8.9%) |
0.632* |
| Fasting Blood Glucose (mg/dL), median (IQR) | 90.7 (38.9) | 111.4 (52.2) | 91.6 (24.5) | - |
|
Lipid profile Total cholesterol (mg/dL), median (IQR) LDLC (mg/dL), median (IQR) Triglycerides (mg/dL), median (IQR) HDLC (mg/dl), median (IQR) Among males Among females Non-HDLC (mg/dL), median (IQR) |
223.5 (92.6) 139.2 (78.8) 140.7 (100.0) 42.3 (18.8) 53.5 (20.4) 167.3 (80.2) |
221.2 (123.4) 126.5 (89.2) 174.3 (114.2) 38.1 (19.5) 43.6 (18.3) 171.1 (111.7) |
225.8 (75.8) 142.9 (60.5) 118.6 (85.8) 46.2 (19.6) 57.6 (18.5) 162.6 (68.1) |
0.383 0.138 - - 0.982 |
|
Blood pressure Systolic blood pressure (mmHg), median (IQR) Diastolic blood pressure (mmHg), median (IQR) |
110 (30) 80 (20) |
120 (30) 80 (20) |
110 (20) 70 (20) |
- - |
Student’s t test
Chi square test
Patients with MS had a waist circumference of 88.4 ± 7.7 in females and 93.3 ± 9.0 cm in males. The median fasting blood glucose was 111.4 (52.2) mg/dL, median systolic blood pressure of 120 (30) mm Hg and diastolic blood pressure of 80 (20) mmHg, median serum triglycerides of 174.3 (114.2) mg/dL, median HDL-C of 42.3 (19.2) mg/dL and a proportion of patients with diabetes (23.0%) and hypertension (44.3%), respectively (Table 1).
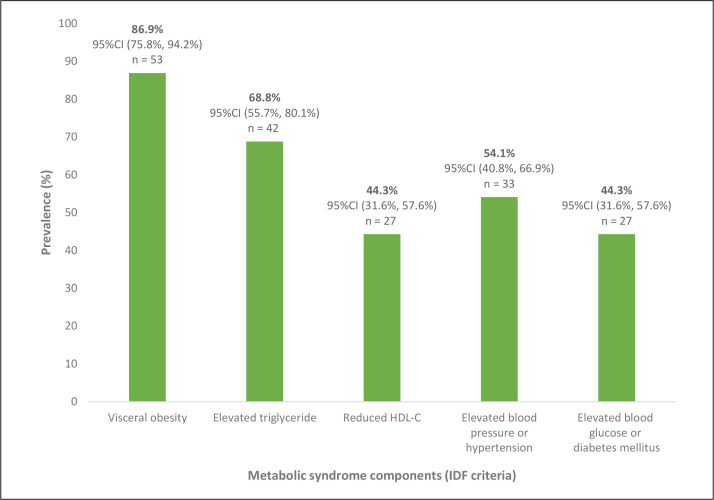
Among the different components of the MS among patients who fulfilled the diagnostic criteria, analysis showed that the component with the highest prevalence was the presence of an increased waist circumference at 86.9% (95%CI: 75.8%, 94.2%), followed by elevated triglyceride levels at 68.8% (95%CI: 55.7%, 80.1%), elevated blood pressure, hypertension or on specific treatment at 54.1% (95%CI: 40.8%, 66.9%), reduced HDL-C levels at 44.3% (95%CI: 31.6%, 57.6%) and elevated blood glucose, diabetes mellitus or on specific treatment at 44.3% (95%CI: 31.6%, 57.6%), respectively (Figure 2).
Figure 2.
Prevalence of each component of metabolic syndrome among those who have satisfied the metabolic syndrome criteria.
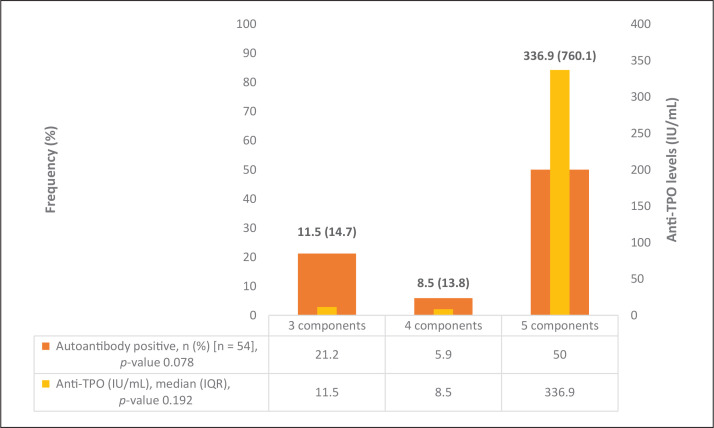
There were no significant differences between those with MS versus those without in terms of age, sex and anti-thyroid peroxidase antibody (anti-TPO) levels (Table 2). The number of components satisfied in the MS criteria in relation to the thyroid function tests (TSH, FT4) and anti-TPO levels are shown in Figure 3. Further analyses of hypothyroid patients diagnosed with MS did not show any significant differences between thyroid function tests and metabolic parameters compared to the etiology of hypothyroidism (Table 3).
Table 2.
Baseline thyroid function tests and comparison of thyroid autoantibody levels between hypothyroid patients with and without metabolic syndrome
| Thyroid tests | (+) Metabolic syndrome (n = 61) | (-) Metabolic syndrome (n = 90) | p |
|---|---|---|---|
|
Thyroid function tests TSH (mIU/mL), median (IQR) FT4 (ng/dL), median (IQR) |
67.8 (90.5) 0.63 (0.93) |
68.0 (72.0) 0.54 (0.64) |
- - |
|
Thyroid autoantibodies Anti-TPO (IU/mL), median (IQR) Autoantibody positive, n (%) |
9.9 (18.8) 10 (18.5%) |
9.4 (9.3) 11 (13.4%) |
0.478* 0.420** |
Student’s t test
Chi square test
Figure 3.
Thyroid autoantibody levels between number of metabolic syndrome components.
Table 3.
Clinical and laboratory characteristics of patients with metabolic syndrome and hypothyroidism
| Post-procedural (n = 44) | Autoimmune (n = 10) | Central (n = 7) | p | |
|---|---|---|---|---|
| Thyroid function tests | ||||
| TSH (mIU/mL), median (IQR) | 69.8 (74.2) | 57.7 (93.8) | 0.1 (0.9) | <0.001* |
| FT4 (ng/dL), median (IQR) | 1.5 (0.2) | 0.6 (0.7) | 0.4 (0.8) | <0.001** |
|
| ||||
| Metabolic parameters | ||||
| Waist circumference (cm), mean (sd) | 90.1 (8.5) | 90.2 (4.2) | 90.1 (9.2) | 0.999# |
| BMI (kg/m2), mean (sd) | 26.2 (3.1) | 24.1 (3.3) | 25.8 (3.9) | 0.391# |
| Total cholesterol (mg/dL), median (IQR) | 230.8 (134.8) | 209.8 (95.5) | 166.5 (92.8) | 0.188# |
| LDLC (mg/dL), median (IQR) | 141.9 (96.7) | 120.5 (87.3) | 97.7 (76.7) | 0.214# |
| Triglycerides (mg/dL), median (IQR) | 192.0 (182.3) | 155.4 (44.1) | 185.1 (66.4) | 0.318# |
| HDLC (mg/dl), median (IQR) | 42.0 (18.1) | 44.0 (24.6) | 38.5 (19.6) | 0.908# |
| Non-HDLC (mg/dL), median (IQR) | 188.4 (121.5) | 161.8 (88.7) | 125.0 (68.8) | 0.152# |
| Fasting blood glucose (mg/dL), median (IQR) | 110.8 (61.5) | 112.7 (30.8) | 102.2 (40.9) | 0.836# |
| Systolic blood pressure (mmHg), median (IQR) | 120 (25) | 130 (20) | 130 (40) | 0.487# |
| Diastolic blood pressure (mmHg), median (IQR) | 80 (20) | 90 (30) | 80 (30) | 0.265# |
Student’s t test
Post-hoc analysis by Dunn test: central is significantly different from autoimmune (p <0.001) and primary non-autoimmune (p <0.001)
Post-hoc analysis by Dunn test: central is significantly different from autoimmune (p <0.001) and primary non-autoimmune (p = 0.001)
DISCUSSION
In this study, we determined the clinical characteristics and prevalence of MS among Filipino adults with hypothyroidism. We found that the prevalence of MS is high at 40.4% (95% CI: 32.5%, 48.7%) among patients with hypothyroidism. This value falls within the available international data on the prevalence of MS among patients with hypothyroidism (Table 4). While our results are consistent with our recent retrospective cross-sectional study conducted earlier in our institution,16 our findings are in contrast to previous locally available data from Cebu City, where the prevalence was only 6.4% among 31 hypothyroid patients. We surmised that this relatively low prevalence of MS among those with hypothyroidism could be from several reasons. First, the exclusion of patients (n = 383) with incomplete data would have led to lesser inclusion of patients with hypothyroidism and subsequently, prevalence. Second, most patients who are stable on thyroid hormone replacement are less likely to seek consultation or follow-up compared to patients with very florid symptoms of hypothyroidism, which could also lead to fewer patients with hypothyroidism included at the onset. Third, as the status of hypothyroidism whether overt, subclinical or treated was not explicitly mentioned, the effect of thyroid hormone replacement on the improvement of metabolic parameters especially weight loss and lipid parameters could have also led to the decrease in prevalence.
Table 4.
Comparison of studies on the prevalence of metabolic syndrome in the general adult population and adults with thyroid disorders
| Author (year) | Country | Population | Prevalence of metabolic syndrome | Reference |
|---|---|---|---|---|
| General Population | ||||
| Noubiap (2022) | Worldwide | N = 28,193,768 adults from 1,129 prevalence studies | 12.5% by NCEP/ATP III criteria 28.2% by IDF criteria 29.1% by NCEP/ATP III-AHA/NHLBI criteria 31.4% by Joint Interim Statement (JIS) criteria |
13 |
| Punzalan (2004) | Philippines | N = 4541 (adults ≥20 years) | 14.2% by NCEP/ATP III criteria 19.3% by International Atherosclerosis (IAS) criteria |
12 |
| Morales (2008) | Philippines | N = 4753 (adults ≥20 years) | 11.9% by NCEP/ATP III criteria 14.5% by IDF criteria 18.6% by NCEP/ATP III-AHA/NHLBI criteria |
14 |
| Mata (2017) | Philippines | N = 1367 | 51.0% by NCEP/ATP III-AHA/NHLBI criteria 29.6% among normal BMI 18.5 – 22.9 kg/m2 38.9% among overweight BMI 23.0 – 24.9 kg/m2 56.9% among obese class I BMI 25.0 – 29.9 kg/m2 62.4% among obese class II BMI ≥ 30 kg/m2 |
15 |
| Patients with thyroid dysfunction | ||||
| Chiu (2023) | Philippines | N = 105 | 36.19% among patients with hypothyroidism using Harmonizing definition (n = 38) 32.43% in men (n = 12) and 38.24% in women |
16 |
| Ogbera (2012) | Nigeria | N = 112 | 28.0% by NCEP/ATP III-AHA/NHLBI criteria 40.0% among patients with hypothyroidism by NCEP/ATP III-AHA/NHLBI criteria (n = 10) 24.0% among patients with hyperthyroidism by NCEP/ATP III-AHA/NHLBI criteria (n = 90) 42.0% among patients with non-toxic goiter by NCEP/ATP III-AHA/NHLBI criteria (n = 12) |
17 |
| Khare (2017) | Southern India | N = 154 | 53.24% among patients with hypothyroidism by NCEP/ATP III criteria (n = 82) | 18 |
| Bermudez (2018) | Venezuela | N = 391 | 56.8% among patients with subclinical hypothyroidism by IDF criteria (n = 41) No data available among euthyroid patients |
19 |
Among the 5 components of MS in the patients who fulfilled the diagnosed criteria, the presence of an increased waist circumference had the highest prevalence at 86.9% (95%CI: 75.8%, 94.2%) while elevated fasting plasma glucose was the least common at 44.3% (95%CI: 31.6%, 57.6%). Our findings are consistent with worldwide data showing that the component with the highest global prevalence is ethnic-specific central obesity at 45.1% (95%CI: 42.1%, 48.2%), while elevated fasting glucose had the least global prevalence at 24.5% (95%CI: 22.5%, 26.6%).13
We also found that patients with central hypothyroidism have significantly higher FT4 levels compared to their primary hypothyroidism counterparts. This finding is consistent with the pathogenesis of central or secondary versus primary hypothyroidism where the primary pathology in the latter is the absent or decreased production of free thyroxine in the thyroid gland in contrast to an intact thyroid hormone synthesis in the former.25-27 However, there were no significant differences between the etiology of hypothyroidism versus anthropometric measurements, clinical and laboratory parameters. This could be explained by the common final pathway of all forms of hypothyroidism which results in an overall decrease in the levels of FT4 and hence, resulting in similar lipid and metabolic abnormalities.25-27 There were also no significant differences between those with MS versus those without in terms of age, sex, and anti-thyroid peroxidase antibody levels. This finding demonstrates that the driving force for the development of MS in a hypothyroid patient is mainly from the end-organ effects of the hypothyroid state itself. Hypothyroidism is characterized as a state of attenuated basal plasma insulin and insulin resistance which can increase cardiovascular risk, especially in the presence of increased waist circumference, increased body mass index, elevated SBP and/or DBP, elevated fasting plasma glucose, serum triglycerides and decreased HDL-C.22
The importance of our study was that we demonstrated that the prevalence of MS among adults with hypothyroidism is high. Our findings provide additional evidence and support early screening of our patients during clinic visits using waist circumference and metabolic parameters to identify patients with hypothyroidism who are at high risk of developing MS. It is interesting to note that screening for MS among patients with hypothyroidism has not yet been recommended as standard of care.28
Our study has several limitations. First, we were limited to a single center. Although we have surpassed our calculated sample size, we could have recruited more patients but we were limited by the prevailing COVID-19 pandemic situation where there was a major drop in the number of outpatient consultations. Second, patients with DM, on glucose-lowering therapy and those with dyslipidemia were included in the study. This might contribute to selection bias potentially leading to overestimation of prevalence. Ideally, patients without comorbidities present in the diagnostic criteria for metabolic syndrome should have been enrolled. Third, we did not include patients with subclinical hypothyroidism as this subset of patients is rarely encountered during consults and is mostly referred to us by other subspecialties when there are incidental thyroid function test result abnormalities. Lastly, we did not look into the effect of hormone replacement therapy and the quantification of the magnitude of changes in terms of anthropometric measurements, blood pressure, fasting blood glucose and serum lipids before and after hormone replacement therapy. The aforementioned limitations can limit the generalizability of our findings yet there can be new avenues for research looking into the effect of treatment on the metabolic profile of patients with hypothyroidism using a larger sample population with the inclusion of patients diagnosed with subclinical hypothyroidism.
CONCLUSION
In summary, our study showed that the prevalence of MS in adult Filipinos with hypothyroidism is high. The presence of increased waist circumference is the most prevalent component seen among hypothyroid patients. Hence, emphasis must be placed on early screening using waist circumference and metabolic parameters among hypothyroid patients who are at high risk of developing MS.
Funding Statement
Funding Source The authors received research grants with a total amount of PhP 250,000: PhP 100,000 from the Philippine Lipid and Atherosclerosis Society and PhP 150,000 from the Philippine College of Endocrinology, Diabetes and Metabolism.
Statement of Authorship
All authors certified fulfillment of ICMJE authorship criteria.
CRediT Author Statement
HHC: Conceptualization, Methodology, Validation, Formal analysis, Investigation, Resources, Data Curation, Writing – original draft preparation, Writing – review and editing, Visualization, Supervision, Project administration, Funding acquisition; EQVIII: Methodology, Validation, Formal analysis, Resources, Data Curation, Writing – original draft preparation, Writing – review and editing, Visualization; RLJ: Methodology, Investigation, Data Curation, Writing – review and editing; AEA: Methodology, Data Curation, Writing – original draft preparation, Writing – review and editing; CJ: Conceptualization, Methodology, Formal analysis, Resources, Data Curation, Writing – review and editing, Supervision, Funding Acquisition.
Author Disclosure
Cecilia Jimeno is the Vice Editor-in-Chief of the JAFES. The rest of the authors declared no conflict of interest.
References
- 1.Peppa M, Betsi G, Dimitriadis G. Lipid abnormalities and cardio-metabolic risk in patients with overt and subclinical thyroid disease. J Lipids. 2011;2011:575840. PMID: 21789282. PMCID: . 10.1155/2011/575840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kim B. Thyroid hormone as a determinant of energy expenditure and the basal metabolic rate. Thyroid. 2008;18(2):141-4. PMID: 18279014. 10.1089/thy.2007.0266. [DOI] [PubMed] [Google Scholar]
- 3.Zhu X, Cheng SY. New insights into regulation of lipid metabolism by thyroid hormone. Curr Opin Endocrinol Diabetes Obes. 2010;17(5): 408-13. PMID: 20644471. PMCID: . 10.1097/MED.0b013e32833d6d46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tagami T, Tamanaha T, Shimazu S, et al. Lipid profiles in the untreated patients with Hashimoto thyroiditis and the effects of thyroxine treatment on subclinical hypothyroidism with Hashimoto thyroiditis. Endocr J. 2010;57(3):253-8. PMID: 20032565. 10.1507/endocrj.k09e-315. [DOI] [PubMed] [Google Scholar]
- 5.Tzotzas T, Krassas GE, Konstantinidis T, Bougoulia M. Changes in lipoprotein(a) levels in overt and subclinical hypothyroidism before and during treatment. Thyroid. 2000;10(9):803-8. PMID: 11041458. 10.1089/thy.2000.10.803. [DOI] [PubMed] [Google Scholar]
- 6.Teixeira Pde F, Reuters VS, Ferreira MM, et al. Lipid profile in different degrees of hypothyroidism and effects of levothyroxine replacement in mild thyroid failure. Transl Res. 2008;151(4):224-31. PMID: 18355770. 10.1016/j.trsl.2007.12.006. [DOI] [PubMed] [Google Scholar]
- 7.Biondi B, Klein I. Hypothyroidism as a risk factor for cardiovascular disease. Endocrine. 2004;24(1):1-13. PMID: 15249698. 10.1385/ENDO:24:1:001. [DOI] [PubMed] [Google Scholar]
- 8.Biondi B, Kahaly GJ. Cardiovascular involvement in patients with different causes of hyperthyroidism. Nat Rev Endocrinol. 2010;6(8): 431-43. PMID: 20585347. 10.1038/nrendo.2010.105. [DOI] [PubMed] [Google Scholar]
- 9.Klein I, Ojamaa K. Thyroid hormone and the cardiovascular system. N Engl J Med. 2001;344(7):501-9. PMID: 11172193. 10.1056/NEJM200102153440707. [DOI] [PubMed] [Google Scholar]
- 10.Fazio S, Palmieri EA, Lombardi G, Biondi B. Effects of thyroid hormone on the cardiovascular system. Recent Prog Horm Res. 2004;59:31-50. PMID: 14749496. 10.1210/rp.59.1.31. [DOI] [PubMed] [Google Scholar]
- 11.Iwen KA, Schröder E, Brabant G. Thyroid hormones and the metabolic syndrome. Eur Thyroid J. 2013;2(2):83-92. PMID: 24783045. PMCID: . 10.1159/000351249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Morales DD, Punzalan FE, Paz-Pacheco E, Sy RG, Duante CA; National Nutrition and health survey: 2003 Group . Metabolic syndrome in the Philippine general population: Prevalence and risk for atherosclerotic cardiovascular disease and diabetes mellitus. Diab Vasc Dis Res. 2008;5(1):36-43. PMID: 18398811. 10.3132/dvdr.2008.007. [DOI] [PubMed] [Google Scholar]
- 13.Noubiap JJ, Nansseu JR, Lontchi-Yimagou E, et al. Geographic distribution of metabolic syndrome and its components in the general adult population: A meta-analysis of global data from 28 million individuals. Diabetes Res Clin Pract. 2022;188:109924. PMID: 35584716. 10.1016/j.diabres.2022.109924. [DOI] [PubMed] [Google Scholar]
- 14.Punzalan FER, Sy RG, Ty-Willing T. Prevalence of metabolic syndrome among adult Filipinos. Int Congr Ser. 2004;1262:442-45. [Google Scholar]
- 15.Mata A, Jasul G Jr. Prevalence of Metabolic Syndrome and its individual features across different (normal, overweight, pre-obese and obese) body mass index (BMI) categories in a tertiary hospital in the Philippines. J ASEAN Fed Endocr. 2017;32(2):117-22. PMID: 33442094. PMCID: . 10.15605/jafes.032.02.04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chiu HHC, Larrazabal Jr. RB, Arcellana AES, Jimeno CA. The prevalence of metabolic syndrome among adult Filipinos with hypothyroidism: A retrospective cohort study. Acta Med Philipp. 2023;57(7):31-7. 10.47895/amp.vi0.4978. [DOI] [Google Scholar]
- 17.Ogbera AO, Kuku S, Dada O. The metabolic syndrome in thyroid disease: A report from Nigeria. Indian J Endocrinol Metab. 2012;16(3): 417-22. PMID: 22629511. PMCID: . 10.4103/2230-8210.95688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Khare J, Nalla S, Wadhwa J, Srivastava P, Reddy B, Deb P. Prevalence of metabolic syndrome in hypothyroidism: Experience in a tertiary center in South India. Chrismed J Health Res. 2017;4(1):19-22. 10.4103/2348-3334.196035. [DOI] [Google Scholar]
- 19.Bermúdez V, Salazar J, Añez R, et al. Metabolic syndrome and subclinical hypothyroidism: A type 2 diabetes-dependent association. J Thyroid Res. 2018;2018:8251076. PMID: 30151097. PMCID: . 10.1155/2018/8251076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Khatiwada S, Sah SK, Kc R, Baral N, Lamsal M. Thyroid dysfunction in metabolic syndrome patients and its relationship with components of metabolic syndrome. Clin Diabetes Endocrinol. 2016;2:3. PMID: 28702239. PMCID: . 10.1186/s40842-016-0021-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Raboca JC, Jimeno CA, Kho SA, et al. The Philippine Thyroid Disease Study (PhilTiDes 1): Prevalence of thyroid disorders among adults in the Philippines. J ASEAN Fed Endocr Soc. 2012;27(1):27-33. 10.15605/jafes.027.01.04. [DOI] [Google Scholar]
- 22.Mehran L, Amouzegar A, Azizi F. Thyroid disease and the metabolic syndrome. Curr Opin Endocrinol Diabetes Obes. 2019;26(5):256-65. PMID: 31369412. 10.1097/MED.0000000000000500. [DOI] [PubMed] [Google Scholar]
- 23.Hsu WC, Araneta MR, Kanaya AM, Chiang JL, Fujimoto W. BMI cut points to identify at-risk Asian Americans for type 2 diabetes screening. Diabetes Care. 2015;38(1):150-8. PMID: 25538311. PMCID: . 10.2337/dc14-2391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Misra A. Ethnic-specific criteria for classification of body mass index: A perspective for Asian Indians and American Diabetes Association Position Statement. Diabetes Technol Ther. 2015;17(9):667-71. PMID: 25902357. PMCID: . 10.1089/dia.2015.0007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Danese D, Sciacchitano S, Gardini A, Andreoli M. Post-operative hypothyroidism. Minerva Endocrinol. 1996;21(3):85-91. PMID: 9072668. [PubMed] [Google Scholar]
- 26.Devdhar M, Ousman YH, Burman KD. Hypothyroidism. Endocrinol Metab Clin North Am. 2007;36(3):595-615. PMID: 17673121. 10.1016/j.ecl.2007.04.008. [DOI] [PubMed] [Google Scholar]
- 27.Gupta V, Lee M. Central hypothyroidism. Indian J Endocrinol Metab. 2011;15(S2):S99-106. PMID: 21966662. PMCID: . 10.4103/2230-8210.83337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jonklaas J, Bianco AC, Bauer AJ, Burman KD, et al. Guidelines for the treatment of hypothyroidism: prepared by the American thyroid association task force on thyroid hormone replacement. Thyroid. 2014;24(12):1670-751. PMID: 25266247. PMCID: . 10.1089/thy.2014.0028. [DOI] [PMC free article] [PubMed] [Google Scholar]