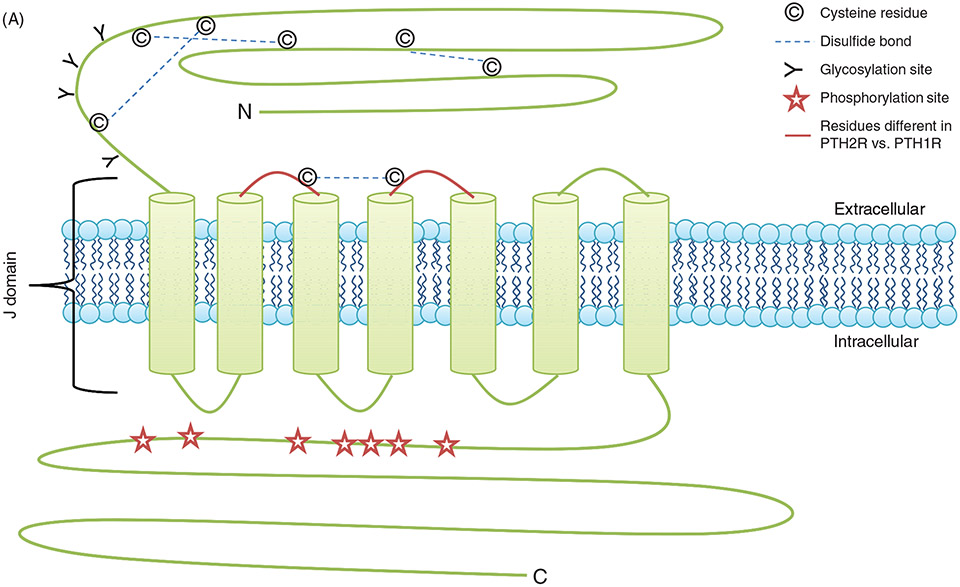
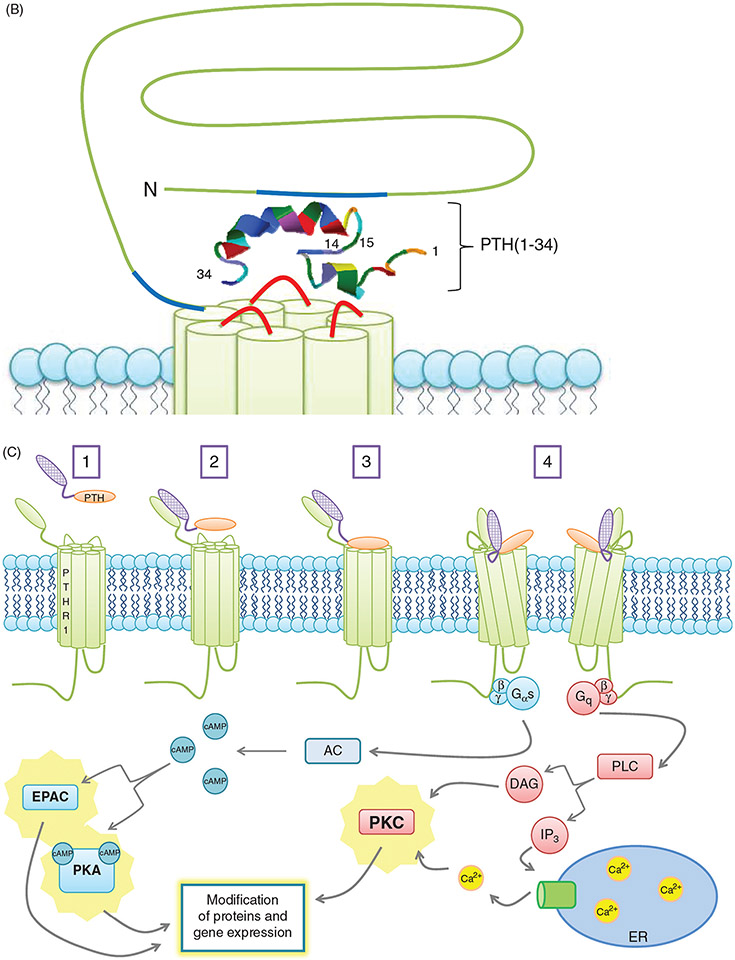
Figure 4.
(A) PTH receptor topology and ligand binding. (A)The PTH1R consists of an extracellular amino terminus, a J domain consisting of transmembrane domains as well as intra- and extracellular loops, and an intracellular carboxy terminus. The extracellular amino terminus is 150 residues long, with 4 N-glycosylation sites and 4 disulfide bridges. Less is known regarding PTH2R topology as compared to PTH1R topology. Importantly, residue variations in two of the extracellular loops (highlighted in red) decrease the affinity of the receptor for PTHrP while maintaining specificity for PTH. (B) PTH receptor topology and ligand binding. (B) PTH(1-34) (rainbow structure) interacts with both the extracellular amino terminus of the PTH1R as well as the J domain. Docking of PTH to the PTH1R is thought to occur through initial binding of the C-terminus of PTH (residues 15-34) to the N-terminus (blue regions) of the PTH1R. This interaction is closely followed by the binding of the N-terminus of PTH (residues 1-14) to the J domain (red regions) of the PTH1R, initiating G protein recruitment and intracellular signaling cascade activation. (C) PTH-receptor binding and intracellular signaling. (1) Two-site model of PTH-receptor docking. (2) The N-domain of the PTH receptor (PTHR1) binds the C-domain of PTH. (3) The J-domain binds the amino-terminal region of PTH. (4) Binding of the ligand to the receptor increases the association with the J-domain, while also increasing the affinity of the intracellular beta-gamma binding region of the C-terminal region of the PTH receptor for G proteins, resulting in their subsequent activation and initiation of downstream signaling cascades. Gαs activates adenylate cyclase (AC), which increases intracellular [cAMP], resulting in activation of Epac and PKA. Gq activates phospholipase C (PLC), forming diacylglycerol (DAG) and inositol triphosphate (IP3). DAG directly activates PKC, whereas IP3 indirectly activates PKC by releasing Ca2+ from the ER.