Abstract
A distinct clinical presentation of respiratory syncytial virus (RSV) infection of humans is bronchiolitis, which has clinical features similar to those of asthma. Substance P (SP), a tachykinin neuropeptide, has been associated with neurogenic inflammation and asthma; therefore, we chose to examine SP-induced inflammation with RSV infection. In this study, we examined the production of pulmonary SP associated with RSV infection of BALB/c mice and the effect of anti-SP F(ab)2 antibodies on the pulmonary inflammatory response. The peak production of pulmonary SP occurred between days 3 and 5 following primary RSV infection and day 1 after secondary infection. Treatment of RSV-infected mice with anti-SP F(ab)2 antibodies suggested that SP may alter the natural killer cell response to primary and secondary infection. In mice challenged after formalin-inactivated RSV vaccination, SP appears to markedly enhance pulmonary eosinophilia as well as increase polymorphonuclear cell trafficking to the lung. Based on studies with a strain of RSV that lacks the G and SH genes, the SP response to RSV infection appears to be associated with G and/or SH protein expression. These data suggest that SP may be an important contributor to the inflammatory response to RSV infection and that anti-SP F(ab)2 antibodies might be used to ameliorate RSV-associated disease.
Respiratory syncytial virus (RSV) is one of most important respiratory pathogens of infants and young children worldwide (12, 24, 28, 29, 35). Acute bronchiolitis is the most distinctive feature of RSV infection in infants and young children, and RSV is the most important cause of bronchiolitis (26, 51, 66). Bronchiolitis is an acute inflammatory process of the respiratory bronchioles leading to symptoms of obstructive airway disease. The clinical presentation of bronchiolitis is similar to that of asthma (48, 51, 64). These similarities have led investigators to consider that the mechanisms underlying RSV bronchiolitis and asthma may be similar (46, 48, 56, 65).
Recent studies have raised the possibility that neurogenic factors, including tachykinins such as substance P (SP), may contribute to pulmonary inflammation associated with asthma (4, 17, 31, 59). For example, SP is active at nanomolar concentrations and has diverse actions including induction of vascular extravasation of immune cells (15, 21), increased adhesion of polymorphonuclear cells and eosinophils to endothelium (36), and potentiation of immune functions of lymphocytes, macrophages, mast cells, and eosinophils (42). SP is thought to act primarily through the specific neurokinin 1 receptor (NK-1R) (3, 9, 10, 43). It is possible that SP can also act in a receptor-independent fashion, because it is a small (11-amino-acid) amphiphilic/amphipathic molecule that can pass through the cellular membrane. The functional relevance of SP in pulmonary inflammation is indicated by studies of NK-1R knockout mice (11). In these studies, immune complexes, which induce vascular permeability and allow infiltration of inflammatory cells into the lungs of normal mice, had no effect in NK-1R knockout mice. These data suggest that SP can initiate immune complex-mediated pulmonary inflammation. These findings were supported by studies which showed a reduction in the magnitude of inflammatory cell recruitment to lungs of antigen-primed mice intratracheally challenged with antigen and given systemic administration of a selective antagonist of NK-1R (32).
The possibility that RSV-mediated bronchiolitis and asthma have similar mechanisms contributing to the disease process suggested to us that a factor such as SP might contribute to both diseases. In this report, we describe studies which examine the induction of SP and anti-SP F(ab)2 antibody (Ab) inhibition of SP activity during RSV infection in mice. We use anti-SP F(ab)2 Ab fragments to avert SP induction by immune complexes. In addition, we took advantage of an RSV strain that lacks the G and SH proteins (33) to determine if either of these proteins contributes to SP-associated inflammation during RSV infection. These studies suggest that SP may have an important role in the inflammatory response to RSV and the G and/or SH protein a role in induction of SP during infection.
MATERIALS AND METHODS
Animals, immunizations, and anti-SP treatment.
Four- to six-week-old, specific-pathogen-free, female BALB/c mice were purchased from Harlan Sprague Dawley Laboratories (Indianapolis, Ind.). The mice were housed in microisolator cages and fed sterilized water and food ad libitum. Mice were anesthetized with Avertin (2,2,2-tribromoethanol) and then intranasally (i.n.) infected with 104 PFU of RSV strain B1 or CP52 diluted in phosphate-buffered saline (PBS; GIBCO Laboratories, Grand Island, N.Y.). Mice were immunized with formalin-inactivated B1 (FI-B1) or FI-parainfluenza virus type 3 (PIV3) with 104 PFU equivalents in the superficial gluteal muscle. All immunized animals were rested >3 weeks prior to challenge. Mice were i.n. challenged with 104 PFU of either B1 or CP52. At various time points postinfection (p.i.), mice were anesthetized and exsanguinated by severing the right caudal artery, and lymphoid organs were removed. All organs were collected on ice in Hanks balanced salt solution. To collect bronchoalveolar lavage (BAL) cells, the lung was lavaged three times in Hanks balanced salt solution containing 1% bovine serum albumin (BSA) (Sigma). No fewer than three mice per treatment were examined per time point.
The anti-SP F(ab)2 treatments were given i.n. at 18 h prior to harvest of immune organs. Either 200 μg of anti-SP F(ab)2 (Accurate Chemical and Scientific Corp., Westbury, N.Y.) or 200 μg of normal mouse F(ab)2 Ab (Jackson ImmunoResearch Laboratories, Inc., West Grove, Pa.) was used for the treatments. F(ab)2 Ab was prepared by 37°C overnight incubation of the Ab in citrate buffer containing 5 μg of pepsin/μg of Ab (Sigma). The Ab was centrifuged at 10,000 × g for 30 min, resuspended in PBS (GIBCO), and run over a protein A column (Sigma) to separate F(ab)2 from Fc Ab fragments.
Viruses.
RSV strains B1 (33) and CP52 (33) and the JS strain of PIV3 were propagated in Vero cells (African green monkey kidney fibroblasts [ATCC CCL 81]) maintained in RPMI 1640 (GIBCO) supplemented with 2% heat-inactivated (56°C) fetal bovine serum (HyClone Laboratories, Salt Lake City, Utah), 1% l-glutamine, and 1% antibiotic-antimycotic (tissue culture medium [TCM]) (all from GIBCO). Upon detectable cytopathic effect, the medium was decanted and replaced with a minimal volume of Dulbecco's modified PBS and frozen at −70°C. The flask was thawed and the loosely adherent cell monolayer was scraped off with a cell scraper (Costar, Cambridge, Mass.) and collected. The cells and supernatant were frozen at −70°C, thawed, and then centrifuged at 2,000 × g for 15 min at 4°C. The titer was determined by methylcellulose plaque assay on Vero or HEp-2 cells. B1 is the parent virus from which CP52 was derived.
Vaccines.
Formalin-inactivated virus was prepared as described previously (62). Briefly, 1 part formalin (Sigma, St. Louis, Mo.) was incubated with 4,000 parts clarified virus lysate (B1, CP52, or PIV3) for 3 days at 37°C and pelleted by centrifugation for 1 h at 50,000 × g. The volume of virus was adjusted to a 1:25 dilution of the original volume in minimal essential medium (GIBCO) and subsequently precipitated with aluminum hydroxide (4 mg/ml; Sigma), resuspended in 1:100 the original volume in serum-free minimal essential medium, and stored at 4°C.
Virus titer in lungs.
The quantities of infectious virus present in individual lung homogenates at various days p.i. or challenge with either B1 or CP52 were determined. Identical weights of individual lung isolates were homogenized in PBS and assayed by plaque assay upon monolayers of Vero cells. Lung titers between B1 and CP52 were similar at day 2 p.i. (mean PFU/g of lung tissue ranged between 350 and 500) but were moderately different at both day 4 p.i. (mean PFU/g of lung tissue ranged between 900 and 1,200 for CP52 and between 1,500 and 2,100 for B1) and day 6 p.i. (mean PFU/g of lung tissue ranged between 200 and 500 for CP52 and between 650 and 1,100 for B1).
Quantitation of SP.
A competitive enzyme-linked immunosorbent assay based on the competition between free SP and a SP tracer for a limited number of SP-specific antibody binding sites was used as suggested by the manufacturer (Cayman Chemical, Ann Arbor, Mich.). Dilutions of BAL were analyzed against an SP standard, and the results were calculated as percent sample or standard bound/maximum bound. Intra- and interassay coefficient of variation was ≤10%.
Flow cytometry.
Single-cell suspensions of BAL cells were blocked with 10% normal mouse serum (Jackson ImmunoResearch Laboratories) in PBS and then stained with the appropriate combinations of fluorescein isothiocyanate- or phycoerythrin-labeled anti-CD3ɛ (145-2C11), anti-CD45R/B220 (RA3-6B2), anti-pan NK cell (DX5), antineutrophil (RB6-8C5), anti-adhesion molecule (CD11b), and mouse isotype Ab controls (all from PharMingen, San Diego, Calif.). A lymphocyte gate was used to select 10,000 events for CD3+ and B220+ lymphocytes; 10,000 ungated events were used for analysis of DX5+, RB6-8C5+, and CD11b+ cells. The distribution of cell surface markers was determined in two-color mode on a FACScan with CellQUEST software (Becton Dickinson, Mountain View, Calif.). The procedure used for intracellular (IC) cytokine staining was modified for microculture staining as described previously (61). Briefly, the IC transport of cytokines was inhibited by culturing cells in PBS containing GolgiStop (PharMingen) for 4 h at 37°C, thereby allowing for accumulation of cytokines in the Golgi of the cells. The cells were washed in PBS (GIBCO), stained with an appropriate dilution of fluorescein isothiocyanate anti-CD3 for 30 min on ice, washed, and resuspended in Cytofix/Cytoperm (PharMingen) for 30 min on ice. The cells were washed in Cytofix/Cytoperm and resuspended in the appropriate dilution of phycoerythrin-labeled anti-interleukin-2 (IL-2) (JES6-5H4), anti-IL-4 (BVD4-1D11), anti-IL-5 (TRFK5), anti-IL-6 (MP5-20F3), or anti-gamma interferon (IFN-γ) (XMG1.2) Ab (all from PharMingen) diluted in PBS containing 1% BSA and 0.1% saponin. The cells were stained on ice for 30 min, washed and resuspended in PBS containing 1% BSA, and analyzed on the FACScan.
Lymphocyte enrichment.
Lymphocytes isolated from three pooled spleens of BALB/c mice were enriched for CD4+ or CD8+ T lymphocytes by using streptavidin-coated magnetic beads (Dynal AS, Oslo, Norway) coupled to biotin-anti-CD8a (53-6.7; PharMingen) or to biotin-anti-CD4 (RM4-5; PharMingen). A portion of the CD4+ and CD8+ T lymphocytes were analyzed by flow cytometry (FACScan; Becton Dickinson) and found to be enriched to >95% with the magnetic beads.
H&E staining of BAL cells.
BAL cells were washed from the lungs of anesthetized mice with PBS containing 0.1% BSA, using a 1-ml syringe and 18-gauge cannula (Baxter, Deerfield, Ill.) as previously described (61). Cells were kept at 4°C, and portions were cytospun onto glass microscope slides, fixed, and stained in hematoxylin and eosin (H&E).
Cell proliferation assay.
Spleen cells from RSV-immune mice were collected and cocultured in triplicate at 106 cells/well in 96-well U-bottom plates (Costar) with TCM, 10−5 or 10−6 M SP, or concanavalin A (CA) at 2 μg/ml. To confirm SP-induced cell proliferation, a portion of the wells received anti-SP antibodies (Accurate) diluted appropriately in TCM to give a final dilution of 1:10, 1:100 or 1:1,000 per well. At day 3 poststimulation, all cells were pulsed with 1 μCi of [3H]thymidine (Amersham, Arlington Heights, Ill.) per well, diluted in TCM for an additional 24 h, then harvested onto fiberglass filters (Cambridge Technologies, Cambridge, Mass.), and analyzed with a Packard beta counter (Meridan, Conn.). The stimulation index represents the mean counts per minute of stimulated cells over the mean counts per minute of unstimulated (TCM) cultures.
RESULTS
SP levels in the BAL specimens.
To determine the level of SP in the BAL associated with acute RSV infection, the level of SP in naive mice was compared to SP levels in mice i.n. infected with either B1 or CP52 (Table 1). The baseline levels of SP in BAL specimens ranged from 200 to 250 pg/ml, and a level of ≥350 pg/ml was always significantly above baseline and therefore considered indicative of an increase in SP levels (Table 1). After primary infection, an increase in SP levels occurred at day 3 p.i., peaked on day 4 p.i., and then decreased to baseline levels by day 5 p.i. for CP52-infected mice and day 6 p.i. for B1-infected mice. The peak SP levels were significantly higher for B1-infected mice. In previously immunized mice, peak SP levels occurred on day 1 p.i. and returned to baseline by day 2 p.i. except for CP52-immunized mice infected with B1. Consistent with the results after primary infection, mice immunized with CP52 and challenged with CP52 had the lowest levels of SP whereas mice immunized with B1 and challenged with B1 had the highest levels of SP in the BAL. These data suggest that G and/or SH proteins contribute to production of SP in the BAL.
TABLE 1.
SP levels in the BAL following infection and challenge with B1 and CP52
| Treatment | Concn (pg/ml) of substance P in BAL
|
|||||
|---|---|---|---|---|---|---|
| Day 1 | Day 2 | Day 3 | Day 4 | Day 5 | Day 6 | |
| None | 220 ± 50 | 250 ± 40 | 250 ± 40 | 200 ± 30 | 250 ± 50 | 240 ± 10 |
| B1 | 200 ± 25 | 220 ± 20 | 450 ± 80 | 800 ± 120 | 550 ± 80 | 450 ± 50 |
| CP52 | 175 ± 30 | 300 ± 60 | 400 ± 50 | 500 ± 20 | 350 ± 50 | 200 ± 50 |
| B1 + B1 | 1,000 ± 90 | 260 ± 50 | NDa | 200 ± 20 | ND | 220 ± 20 |
| B1 + CP52 | 700 ± 50 | 175 ± 20 | ND | 250 ± 60 | ND | 220 ± 50 |
| CP52 + B1 | 750 ± 60 | 400 ± 100 | 220 ± 50 | ND | 200 ± 40 | ND |
| CP52 + CP52 | 500 ± 30 | 250 ± 20 | 200 ± 50 | ND | 250 ± 50 | ND |
| FI-B1 | 180 ± 20 | 140 ± 20 | ND | 200 ± 50 | ND | 220 ± 20 |
| FI-CP52 | 120 ± 20 | 140 ± 50 | ND | 150 ± 20 | ND | 200 ± 20 |
| FI-B1 + B1 | 1,200 ± 120 | 800 ± 80 | 450 ± 120 | ND | 300 ± 90 | ND |
| FI-B1 + CP52 | 410 ± 100 | 200 ± 60 | 225 ± 20 | ND | 250 ± 80 | ND |
| FI-CP52 + B1 | 600 ± 150 | 220 ± 20 | 225 ± 50 | ND | 200 ± 50 | ND |
| FI-CP52 + CP52 | 600 ± 150 | 180 ± 50 | 150 ± 50 | ND | 200 ± 20 | ND |
ND, not determined.
The possibility that G and/or SH is important to SP production after RSV infection was further supported by experiments with mice immunized with formalin-inactivated vaccine (Table 1). In these experiments, there was a marked increase in peak levels and persistence of higher levels of SP in BAL specimens of mice immunized and challenged with B1 containing the G and SH proteins. The peak SP levels were ≥2-fold higher than that for other groups and were elevated through day 3. When CP52 was used for the formalin-inactivated immunization or challenge or both, SP levels were elevated only on day 1.
Inhibition of SP with anti-SP Ab.
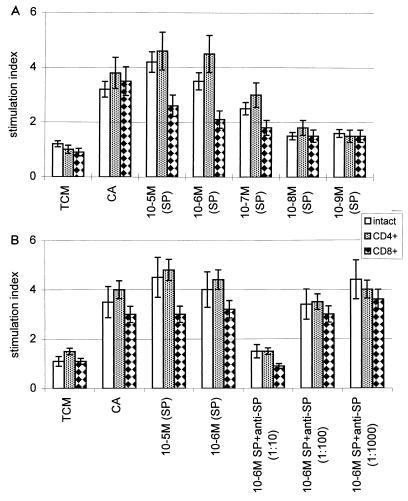
To determine the effect of SP on the lymphocyte compartment, unseparated (intact) cells and CD4+ and CD8+ T cells isolated from the spleens of RSV-immune mice were cocultured with various concentrations of SP or CA (Fig. 1A). Unseparated cells, CD4+ cells, and CD8+ T cells proliferated extensively during coculture with 10−5 to 10−7 M SP; however, within the T-cell population, CD4+ T-cell proliferation was more remarkable. Cell proliferation by all cell subsets was minimal at 10−8 and 10−9 M SP but was statistically above the level of proliferation observed for coculture with TCM. Proliferative responses by spleen cells from naive mice revealed that minimal cell proliferation occurred in response to SP for both unseparated cells and CD4+ and CD8+ T cells (data not shown). This result suggests that activated CD4+ T cells are more responsive to SP in vitro.
FIG. 1.
SP-induced cell proliferation or inhibition of SP-induced cell proliferation by in vitro addition of anti-SP F(ab)2 Ab. (A) Intact spleen cells and CD4+- or CD8+-enriched RSV-immune T cells were cocultured with TCM, CA, or various doses of SP. The proliferation values are given as a stimulation index determined by dividing the mean experimental proliferation value by the mean of the TCM control proliferation value. (B) Intact spleen cells and CD4+- or CD8+-enriched RSV-immune T cells were cocultured with TCM, CA, or various doses of SP and anti-SP F(ab)2 Ab. The proliferation values are given as a stimulation index determined by dividing the mean experimental proliferation value by the mean of the TCM control proliferation value. The addition of either a 1:10 dilution of anti-SP F(ab)2 or nIg F(ab)2 Ab to CA-stimulated cultures did not affect the stimulation index (data not shown).
To determine if anti-SP Abs could block SP-mediated cell proliferation, we cocultured RSV-immune cells with anti-SP Ab and determined the level of proliferation (Fig. 1B). Three dilutions (1:10, 1:100, and 1:1,000) of anti-SP Ab were added to cultures of either unseparated cells or CD4+ or CD8+ T cells cocultured with 10−6 M SP. A 1:10 dilution of anti-SP Ab added to the cells at the beginning of culture fully inhibited SP-mediated proliferation. A dose-responsive inhibition of proliferation was observed. This result showed that lymphocyte proliferation mediated by SP was inhibited by anti-SP antibody and that the inhibitory effect is not limited to a particular subset of T cells.
Anti-SP treatment decreases RSV-associated pulmonary inflammation.
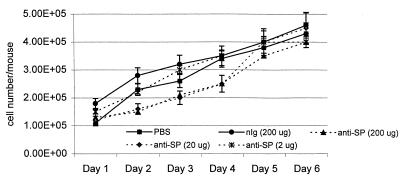
Since we were able to inhibit SP in vitro with anti-SP Ab, we chose to use anti-SP F(ab)2 antibodies to block the actions of SP in vivo, thereby allowing us to examine the contribution of SP to the pulmonary inflammatory response to RSV. To determine the effect of anti-SP Ab treatment on cell numbers in the lungs of mice during primary infection by B1, mice were administered either PBS, 200 μg of F(ab)2 normal immunoglobulin (nIg) Ab, or various doses of anti-SP F(ab)2 Ab 24 h prior to harvest of the BAL (Fig. 2). BAL cell numbers were similar following treatment with PBS, nIg, or 2 μg of anti-SP Ab but were significantly lower at days 2, 3, and 4 p.i. for mice treated with 20 or 200 μg of anti-SP Ab. By days 5 and 6 p.i., the cell numbers were similar between groups. These data suggest that anti-SP Ab treatment alters cell trafficking to the lung during the early (i.e., days 2 to 4 p.i.) phase of the immune response to RSV.
FIG. 2.
Effect of PBS, nIg F(ab)2 Ab, or various doses of anti-SP F(ab)2 Ab on the total cell number in the lungs of mice following acute RSV B1 infection.
To determine if anti-SP Ab treatment altered the recruitment of a particular cell type to the lung, H&E staining of BAL cells was used following infection with either B1 or CP52 and treatment with anti-SP F(ab)2 or nIg F(ab)2 Ab (Table 2). The cell profiles in the BAL of mice infected with B1 and treated with either nIg F(ab)2 or anti-SP F(ab)2 Ab were similar at days 2 and 4 p.i. (Table 2). At these time points, the primary cell types in the BAL were macrophages, followed by lymphocytes, polymorphonuclear leukocytes and eosinophils.
TABLE 2.
BAL cell types by H&E staining and by flow cytometry at days 2 and 4 after infection with B1 or CP52 and treatment with nIg F(ab)2 or anti-SP F(ab)2 Ab
| Type of analysis | Challenge + treatment | Cell typeb | Day 2 p.i.
|
Day 4 p.i.
|
||
|---|---|---|---|---|---|---|
| Range, % positive | Median % (P value)a | Range, % positive | Median % (P value)a | |||
| H&E staining | B1 + nIg | Macrophage | 85–95 | 89 (>0.10) | 80–93 | 85 (>0.10) |
| PMN | 0–4 | 2 (>0.10) | 0–4 | 3 (>0.10) | ||
| Eosinophil | 1–5 | 4 (>0.10) | 0–2 | 2 (>0.10) | ||
| Lymphocyte | 4–8 | 5 (>0.10) | 2–12 | 10 (>0.10) | ||
| B1 + anti-SP | Macrophage | 88–98 | 94 | 78–94 | 85 | |
| PMN | 0 | 0 | 1–5 | 2 | ||
| Eosinophil | 0 | 0 | 0–6 | 2 | ||
| Lymphocyte | 5–14 | 6 | 8–15 | 11 | ||
| CP52 + nIg | Macrophage | 80–95 | 88 (>0.10) | 88–96 | 90 (>0.10) | |
| PMN | 0–4 | 3 (>0.10) | 0–6 | 2 (>0.10) | ||
| Eosinophil | 0 | 0 (>0.10) | 1–5 | 2 (>0.10) | ||
| Lymphocyte | 6–12 | 9 (>0.10) | 4–10 | 6 (>0.10) | ||
| CP52 + anti-SP | Macrophage | 90–98 | 94 | 80–94 | 88 | |
| PMN | 0 | 0 | 1–6 | 2 | ||
| Eosinophil | 0 | 0 | 0–6 | 2 | ||
| Lymphocyte | 4–15 | 6 | 5–12 | 8 | ||
| Flow cytometry | B1 + nIg | CD4 | 18–25 | 20 (>0.10) | 19–25 | 22 (>0.10) |
| CD8 | 6–12 | 8 (>0.10) | 8–15 | 12 (>0.10) | ||
| B220 | 6–10 | 7 (>0.10) | 4–6 | 5 (>0.10) | ||
| DX5 | 4–9 | 6 (<0.01) | 4–8 | 6 (<0.01) | ||
| RB6-8C5 | 4–6 | 4 (>0.10) | 9–14 | 12 (>0.10) | ||
| B1 + anti-SP | CD4 | 15–20 | 16 | 21–28 | 25 | |
| CD8 | 6–8 | 6 | 6–12 | 8 | ||
| B220 | 2–6 | 5 | 4–7 | 6 | ||
| DX5 | 10–17 | 12 | 15–24 | 19 | ||
| RB6-8C5 | 4–6 | 5 | 6–12 | 8 | ||
| CP52 + nIg | CD4 | 19–25 | 24 (>0.10) | 25–30 | 28 (>0.10) | |
| CD8 | 9–15 | 10 (>0.10) | 10–15 | 12 (>0.10) | ||
| B220 | 4–6 | 4 (>0.10) | 3–6 | 4 (>0.10) | ||
| DX5 | 14–18 | 15 (>0.01) | 18–24 | 20 (>0.10) | ||
| RB6-8C5 | 8–14 | 10 (<0.01) | 15–20 | 18 (<0.01) | ||
| CP52 + anti-SP | CD4 | 16–21 | 20 | 20–30 | 26 | |
| CD8 | 5–12 | 8 | 5–14 | 10 | ||
| B220 | 2–6 | 4 | 2–8 | 5 | ||
| DX5 | 8–14 | 10 | 15–20 | 18 | ||
| RB6-8C5 | 4–10 | 5 | 6–12 | 10 | ||
For comparison between nIg and anti-SP-treated mice given the same challenge virus.
A lymphocyte gate was used to select 10,000 events for CD3+ and B220+ lymphocytes; 10,000 ungated events were used for analysis of DX5+, RB6-8C5+, and CD11b+ cells.
Examination of the cell surface markers on the BAL cells identified differences between anti-SP treatment and nIg treatment for B1- and CP52-infected mice after primary infection (Table 2). There was a significant increase in the percent of DX5+ cells after anti-SP treatment in B1-infected mice to levels similar to those seen in both groups of CP52-infected mice. There was no significant change associated with anti-SP treatment for other cell types for B1-infected or cell types for CP52-infected mice. As noted previously (61), CP52-infected mice had significantly increased numbers of DX5+ and/or RB6-8C5+ cells compared to B1-infected mice. These data suggest that SP can alter the trafficking of polymorphonuclear (RB6-8C5+) and NK (DX5+) cells to the lung during the inflammatory response to RSV, and this effect is in part associated with the presence of the G and/or SH proteins.
Anti-SP ameliorates enhanced pulmonary inflammation in FI-RSV-immune mice challenged with RSV.
RSV challenge of BALB/c mice immunized with FI-RSV results in enhanced pulmonary inflammation characterized by eosinophilia and a robust granular cell infiltrate (49, 62). To elucidate the role of SP in enhanced disease, FI-B1-immune mice were challenged with either B1 or CP52 and subsequently treated with nIg F(ab)2 or anti-SP F(ab)2 Ab (Table 3). As a control, FI-PIV3-immune mice were similarly challenged with either B1 or CP52 (Table 4). Administration of anti-SP compared to administration of nIg to FI-B1-immune mice challenged with B1 dramatically altered the pulmonary cell infiltrate. In the BAL, the percentage of macrophages increased approximately twofold, whereas the percentages of PMN and eosinophils decreased approximately 2- and threefold at days 2 and 4 p.i. (Table 3). This pattern of pulmonary cell infiltrate was very similar to that for FI-B1-immune mice challenged with CP52 (Table 3). Administration of anti-SP F(ab)2 Ab had no significant impact on the cell profile in FI-B1-immune mice challenged with CP52. These data demonstrate that anti-SP F(ab)2 Ab treatment can inhibit eosinophilia and PMN infiltration associated with FI-RSV vaccination.
TABLE 3.
BAL cell types by H&E staining and by flow cytometry at days 2 and 4 after B1 or CP52 challenge of FI-RSV-immune mice treated with nIg F(ab)2 or anti-SP F(ab)2 Ab
| Type of analysis | Immunization + challenge + treatment | Cell typeb | Day 2 p.i.
|
Day 4 p.i.
|
||
|---|---|---|---|---|---|---|
| Range, % positive | Median % (P value)a | Range, % positive | Median % (P value) | |||
| H&E staining | FI-B1 + B1 + nIg | Macrophage | 38–50 | 44 (<0.01) | 40–53 | 46 (<0.01) |
| PMN | 15–20 | 18 (<0.01) | 6–15 | 10 (<0.01) | ||
| Eosinophil | 19–30 | 28 (<0.01) | 24–38 | 30 (<0.01) | ||
| Lymphocyte | 8–15 | 10 (<0.01) | 10–22 | 14 (>0.10) | ||
| FI-B1 + B1 + anti-SP | Macrophage | 88–96 | 87 | 70–84 | 75 | |
| PMN | 0 | 0 | 2–11 | 5 | ||
| Eosinophil | 2–12 | 8 | 4–16 | 10 | ||
| Lymphocyte | 4–12 | 5 | 2–12 | 10 | ||
| FI-B1 + CP52 + nIg | Macrophage | 88–98 | 91 (>0.10) | 78–90 | 82 (>0.10) | |
| PMN | 0–1 | 1 (>0.10) | 0–10 | 5 (>0.10) | ||
| Eosinophil | 0–5 | 3 (>0.10) | 1–5 | 5 (>0.10) | ||
| Lymphocyte | 2–10 | 5 (>0.10) | 6–12 | 8 (>0.10) | ||
| FI-B1 + CP52 + anti-SP | Macrophage | 90–96 | 95 | 78–94 | 88 | |
| PMN | 0 | 0 | 0–2 | 2 | ||
| Eosinophil | 0 | 0 | 0–5 | 2 | ||
| Lymphocyte | 3–12 | 5 | 2–10 | 8 | ||
| Flow cytometry | FI-B1 + B1 + nIg | CD4 | 18–25 | 24 (>0.10) | 22–28 | 26 (>0.10) |
| CD8 | 5–8 | 10 (>0.10) | 5–10 | 8 (>0.10) | ||
| B220 | 5–10 | 7 (>0.10) | 3–5 | 3 (>0.10) | ||
| DX5 | 5–12 | 8 (<0.01) | 5–18 | 10 (<0.01) | ||
| RB6-8C5 | 5–10 | 6 (>0.10) | 7–12 | 10 (>0.10) | ||
| FI-B1 + B1 + anti-SP | CD4 | 18–26 | 22 | 21–26 | 24 | |
| CD8 | 8–10 | 10 | 5–11 | 8 | ||
| B220 | 3–6 | 5 | 2–5 | 5 | ||
| DX5 | 14–20 | 17 | 14–25 | 20 | ||
| RB6-8C5 | 2–10 | 6 | 5–12 | 10 | ||
| FI-B1 + CP52 + nIg | CD4 | 22–28 | 24 (>0.10) | 26–30 | 28 (>0.10) | |
| CD8 | 5–10 | 8 (>0.10) | 5–14 | 10 (>0.10) | ||
| B220 | 6–8 | 6 (<0.05) | 4–8 | 6 (>0.10) | ||
| DX5 | 14–20 | 15 (>0.10) | 18–25 | 20 (>0.10) | ||
| RB6-8C5 | 12–20 | 14 (<0.01) | 20–25 | 24 (<0.01) | ||
| FI-B1 + CP52 + anti-SP | CD4 | 17–22 | 21 | 25–28 | 28 | |
| CD8 | 6–12 | 10 | 10–15 | 12 | ||
| B220 | 8–12 | 10 | 6–12 | 8 | ||
| DX5 | 10–18 | 14 | 17–20 | 18 | ||
| RB6-8C5 | 5–10 | 8 | 6–10 | 9 | ||
Comparison between nIg and anti-SP-treated mice given the same challenge virus.
A lymphocyte gate was used to select 10,000 events for CD3+ and B220+ lymphocytes; 10,000 ungated events were used for analysis of DX5+, RB6-8C5+, and CD11b+ cells.
TABLE 4.
BAL cell types by H&E staining and by flow cytometry at days 2 and 4 post-B1 or -CP52 challenge of FI-PIV3-immune mice treated with nIg F(ab)2 or anti-SP F(ab)2 antibodies
| Type of analysis | Immunization + challenge + treatment | Cell typeb | Day 2 p.i.
|
Day 4 p.i.
|
||
|---|---|---|---|---|---|---|
| Range, % positive | Median % (P value)a | Range, % positive | Median % (P value) | |||
| H&E staining | FI-PIV3 + B1 + nIg | Macrophage | 80–92 | 90 (>0.10) | 81–97 | 88 (>0.10) |
| PMN | 0–6 | 4 (>0.10) | 5–10 | 4 (>0.10) | ||
| Eosinophil | 0–3 | 1 (>0.10) | 2–4 | 2 (>0.10) | ||
| Lymphocyte | 2–10 | 5 (<0.05) | 5–10 | 6 (<0.01) | ||
| FI-PIV3 + B1 + anti-SP | Macrophage | 80–95 | 87 | 84–90 | 85 | |
| PMN | 0 | 0 | 0–8 | 4 | ||
| Eosinophil | 2–5 | 3 | 0–4 | 1 | ||
| Lymphocyte | 5–15 | 10 | 10–14 | 10 | ||
| FI-PIV3 + CP52 + nIg | Macrophage | 90–98 | 96 (>0.10) | 90–98 | 96 (<0.01) | |
| PMN | 0 | 0 (>0.10) | 0–3 | 2 (>0.10) | ||
| Eosinophil | 0 | 0 (>0.10) | 0 | 0 (>0.10) | ||
| Lymphocyte | 2–8 | 4 (>0.10) | 2–4 | 2 (<0.01) | ||
| FI-PIV3 + CP52 + anti-SP | Macrophage | 88–98 | 95 | 80–88 | 84 | |
| PMN | 0 | 0 | 4–12 | 6 | ||
| Eosinophil | 0 | 0 | 1–5 | 2 | ||
| Lymphocyte | 4–10 | 5 | 6–14 | 8 | ||
| Flow cytometry | FI-PIV3 + B1 + nIg | CD4 | 20–28 | 26 (>0.10) | 25–34 | 28 (>0.10) |
| CD8 | 10–15 | 12 (>0.10) | 15–21 | 18 (<0.01) | ||
| B220 | 4–10 | 5 (>0.10) | 9–14 | 10 (<0.01) | ||
| DX5 | 2–8 | 5 (<0.01) | 0–8 | 5 (<0.01) | ||
| RB6-8C5 | 2–8 | 4 (>0.10) | 2–10 | 5 (>0.10) | ||
| FI-PIV3 + B1 + anti-SP | CD4 | 20–28 | 25 | 18–30 | 26 | |
| CD8 | 8–20 | 15 | 10–15 | 12 | ||
| B220 | 3–6 | 4 | 2–10 | 5 | ||
| DX5 | 8–18 | 14 | 14–25 | 18 | ||
| RB6-8C5 | 2–11 | 7 | 5–10 | 6 | ||
| FI-PIV3 + CP52 + nIg | CD4 | 18–25 | 22 (>0.10) | 20–28 | 25 (>0.10) | |
| CD8 | 8–15 | 10 (>0.10) | 10–16 | 14 (>0.10) | ||
| B220 | 4–10 | 8 (>0.10) | 4–8 | 5 (>0.10) | ||
| DX5 | 15–21 | 18 (>0.10) | 20–26 | 24 (>0.10) | ||
| RB6-8C5 | 10–16 | 15 (<0.01) | 10–22 | 19 (>0.10) | ||
| FI-PIV3 + CP52 + anti-SP | CD4 | 21–28 | 26 | 22–32 | 26 | |
| CD8 | 6–18 | 8 | 8–20 | 15 | ||
| B220 | 4–10 | 5 | 4–9 | 5 | ||
| DX5 | 14–18 | 15 | 20–25 | 22 | ||
| RB6-8C5 | 4–12 | 8 | 12–20 | 15 | ||
For comparison between nIg and anti-SP-treated mice given the same challenge virus.
A lymphocyte gate was used to select 10,000 events for CD3+ and B220+ lymphocytes; 10,000 ungated events were used for analysis of DX5+, RB6-8C5+, and CD11b+ cells.
The BAL cell surface phenotypes were examined from FI-RSV-immune mice treated with anti-SP or nIg antibody (Table 3). Administration of anti-SP Ab compared to nIg to FI-B1-immune mice challenged with B1 resulted in an increase in the percentage of cells positive for DX5+ cells and to a level similar to that observed following challenge with CP52 (Table 3). The percentage of cells positive for other surface markers was unaltered. This increase in DX5+ cells is comparable to that noted earlier with primary and secondary infection with B1 (Table 2) and is also seen in mice immunized with FI-PIV3 and challenged with B1 (Table 4). Administration of anti-SP Ab had some effect on FI-B1-immunized mice challenged with CP52. The percentage of RB6-8C5+ cells was significantly decreased. A similar effect was observed at day 2 p.i. in FI-PIV3-immune mice challenged with CP52 (Table 4). The percentages of cells positive for other cell surface markers were similar for FI-B1-immune mice challenged with CP52 and was not affected by administration of anti-SP F(ab)2 Ab. These data suggest that SP can alter trafficking of NK cells and PMN, an effect in part associated with G and/or SH proteins.
Anti-SP Ab treatment suppresses IC cytokine expression by CD3+ T lymphocytes.
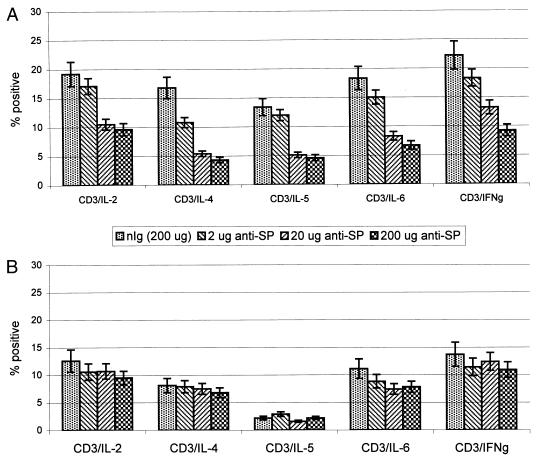
To address one of the mechanisms by which anti-SP F(ab)2 Ab treatment may modify the BAL cell profile, we examined the IC cytokine profiles of pulmonary CD3+ T lymphocytes during the peak (day 5 p.i.) of the acute response to B1 infection (Fig. 3). Mice were administered various doses of anti-SP F(ab)2 Ab or treated with a single 200-μg dose of nIg F(ab)2 antibody and examined at 18 h (Fig. 3A) or 36 h (Fig. 3B) posttreatment for Th1 or Th2 cytokine expression. At 18 h posttreatment, a marked dose-responsive reduction in IL-2, IL-4, IL-5, IL-6, and IFN-γ expression occurred for mice given 20 or 200 μg of anti-SP F(ab)2 Ab (Fig. 3A). Less of an effect was observed for mice treated with 2 μg of anti-SP F(ab)2 Ab compared to nIg F(ab)2 Ab treatment. The effects of anti-SP F(ab)2 Ab treatment were not long lasting, as there were no differences observed between anti-SP F(ab)2 and nIg F(ab)2 Ab treatment at 36 h posttreatment (Fig. 3B). It is likely that the anti-SP antibody is rapidly cleared from the lung following treatment.
FIG. 3.
In vivo anti-SP Ab treatment suppresses IC cytokine expression by CD3+ T lymphocytes. (A) Mice were treated day 5 after RSV infection with doses of anti-SP F(ab)2 Ab or nIg F(ab)2 Ab; the BAL cells were recovered 18 h posttreatment and analyzed for IC cytokine expression. (B) Mice were treated day 5 after RSV infection with doses of anti-SP F(ab)2 Ab or nIg F(ab)2 Ab; the BAL cells were recovered 36 h posttreatment and analyzed for IC cytokine expression.
DISCUSSION
The results of this study suggest that SP has an important role in pulmonary inflammation mediated by RSV infection. We show that SP is produced in the lungs of RSV-infected BALB/c mice, and inhibition of SP with anti-SP F(ab)2 Ab alters the inflammatory cell infiltrate and expression of cytokines by T lymphocytes. The effect of anti-SP F(ab)2 Ab on the BAL infiltrate suggests that SP can inhibit trafficking of DX5+ NK cells to the site of infection and, in the absence of G and/or SH proteins (CP52), increase the number of RB6-8C5+ PMN during primary and secondary RSV infection. The role of SP appears to be even greater in the enhanced inflammatory response associated with FI-RSV vaccination. The administration of anti-SP F(ab)2 antibody was associated with a marked alteration in the type of inflammatory cells in the BAL. This alteration suggests that SP can induce a marked increase in eosinophils and PMN during RSV infection of FI-RSV-immune mice. Treatment with anti-SP F(ab)2 Ab decreased the percentage of cells positive for some IC cytokines, suggesting that SP may in part alter the inflammatory response by altering the expression of cytokines.
The fact that anti-SP F(ab)2 Ab had little effect on the inflammatory response in mice challenged with the CP52 virus suggests that the G and/or SH proteins have a substantial role in the induction of SP during RSV infection. Although our data do not directly implicate the G protein since CP52 lacks the genes for both the G and SH proteins, we suspect the G protein is most likely to affect SP production. The G protein is becoming a key focus in studies of pathogenesis of RSV disease. Previous studies have suggested that the G protein expressed in a vaccinia virus construct can prime for pulmonary eosinophilia (5, 30), and several G protein peptides have been linked to the induction of eosinophilia as well. Several studies have shown that a polarized Th2-type immune response can be induced by priming with the G protein (18, 23, 30, 62) and more specifically with the region spanning residues 193 to 203 (53) or 183 to 197 (58) of the G protein. In part contradictory but in line with studies showing that live virus primes for both Th1- and Th2-type responses, the 183–197 region of G has been shown to induce both IL-5 and IFN-γ responses in G-primed mice challenged with RSV (58). It appears that the Th2 cytokine response to this region may be down regulated by CD8+ T cells (54). A recent study suggests that the cytokine response to this epitope is major histocompatibility complex independent (55) and that differences in the cytokine response may reflect different T-cell reactivities for this region. The results from the present study suggest that SP may be a major factor in the G-protein-induced inflammatory response to RSV infection, and this could explain a major histocompatibility complex-independent response. SP is a neuropeptide primarily secreted from afferent neurons and is likely not affected by cytokine production. By utilizing anti-SP F(ab)2 antibodies to inhibit SP produced during infection or challenge with B1 or CP52, the present study links SP to the apparent G and/or SH protein inhibition of DX5+ (NK) cells in pulmonary inflammation and enhanced disease. The data reveal that anti-SP F(ab)2 Ab treatment can decrease trafficking to the lung of eosinophils and PMNs in FI-RSV-immunized mice challenge with RSV. The basis of this link is under further investigation. It is likely that SP is an important and previously unappreciated contributor to the inflammatory response to RSV.
Assessing the presence of tachykinins (or SP) and characterizing their effects on cells of the immune system has been the object of several studies that have suggested that SP might alter the immune response (2, 16, 42, 45, 47). Several studies have reported that SP facilitates lymphocyte proliferation in response to mitogenic stimulation (1, 19) and increases cytokine production (6, 7, 20, 22, 36–38, 40, 44, 50). SP has also been shown to regulate macrophage function such as superoxide anion production (8, 14, 39, 57). Macrophages themselves express preprotachykinin mRNA (27, 34), suggesting that they may produce SP. Tachykinins, such as SP, have been shown to stimulate production of proinflammatory cytokines such as IL-1, IL-6, and tumor necrosis factor alpha by monocytes and macrophages (6, 7, 20, 22, 36–38, 40, 44, 50). Interestingly, eosinophils in Schistosoma granulomas were shown to produce SP and that the T cells infiltrating the granulomas had upregulated the SP-specific NK1 receptors (63). It is intriguing to speculate that SP may also be produced during eosinophilia associated with RSV-mediated enhanced in mice and in asthma in humans. There are a few studies which have examined tachykinins for their role in human diseases such as rheumatoid arthritis (13, 25) and asthma (4, 31, 41, 52). However, the relationship of SP and pathology in these diseases has yet to be fully appreciated.
It appears that SP contributes to the inflammatory response mediated by RSV infection, that the G and/or SH proteins are important to the induction of the SP-mediated response, and that a number of different immune cells cooperate in the SP-associated inflammatory response. Our finding that a single dose of anti-SP F(ab)2 Ab is sufficient to alter inflammation associated with an ongoing RSV infection suggests a possible therapeutic use for anti-SP Ab in RSV and possibly other inflammatory diseases.
REFERENCES
- 1.Agro A, Stanisz A M. Inhibition of murine intestinal inflammation by anti-substance P antibody. Reg Immunol. 1993;5:120–126. [PubMed] [Google Scholar]
- 2.Agro A, Stanisz A M. Neuroimmunomodulation: classical and non-classical cellular activation. Adv Neuroimmunol. 1995;5:311–319. doi: 10.1016/0960-5428(95)00018-w. [DOI] [PubMed] [Google Scholar]
- 3.Bai T R, Zhou D, Weir T, Walker B, Hegele R, Hayashi S, McKay K, Bondy G P, Fong T. Substance P (NK1)- and neurokinin A (NK2)-receptor gene expression in inflammatory airway diseases. Am J Physiol. 1995;269:L309–L317. doi: 10.1152/ajplung.1995.269.3.L309. [DOI] [PubMed] [Google Scholar]
- 4.Barnes P J. Neurogenic inflammation in airways. Int Arch Allergy Appl Immunol. 1991;94:303–309. doi: 10.1159/000235392. [DOI] [PubMed] [Google Scholar]
- 5.Bembridge G P, Garcia-Beato R, Lopez J A, Melero J A, Taylor G. Subcellular site of expression and route of vaccination influence pulmonary eosinophilia following respiratory syncytial virus challenge in BALB/c mice sensitized to the attachment G protein. J Immunol. 1998;161:2473–2480. [PubMed] [Google Scholar]
- 6.Berczi I, Chalmers I M, Nagy E, Warrington R J. The immune effects of neuropeptides. Baillieres Clin Rheumatol. 1996;10:227–257. doi: 10.1016/s0950-3579(96)80016-1. [DOI] [PubMed] [Google Scholar]
- 7.Berman A S, Chancellor-Freeland C, Zhu G, Black P H. Substance P primes murine peritoneal macrophages for an augmented proinflammatory cytokine response to lipopolysaccharide. Neuroimmunomodulation. 1996;3:141–149. doi: 10.1159/000097239. [DOI] [PubMed] [Google Scholar]
- 8.Boichot E, Lagente V, Paubert-Braquet M, Frossard N. Inhaled substance P induces activation of alveolar macrophages and increases airway responses in the guinea-pig. Neuropeptides. 1993;25:307–313. doi: 10.1016/0143-4179(93)90048-f. [DOI] [PubMed] [Google Scholar]
- 9.Bowden J J, Baluk P, Lefevre P M, Vigna S R, McDonald D M. Substance P (NK1) receptor immunoreactivity on endothelial cells of the rat tracheal mucosa. Am J Physiol. 1996;270:L404–L414. doi: 10.1152/ajplung.1996.270.3.L404. [DOI] [PubMed] [Google Scholar]
- 10.Bowden J J, Garland A M, Baluk P, Lefevre P, Grady E F, Vigna S R, Bunnett N W, McDonald D M. Direct observation of substance P-induced internalization of neurokinin 1 (NK1) receptors at sites of inflammation. Proc Natl Acad Sci USA. 1994;91:8964–8968. doi: 10.1073/pnas.91.19.8964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bozic C R, Lu B, Hopken U E, Gerard C, Gerard N P. Neurogenic amplification of immune complex inflammation. Science. 1996;273:1722–1725. doi: 10.1126/science.273.5282.1722. [DOI] [PubMed] [Google Scholar]
- 12.Brandt C D, Kim H W, Arrobio J O, Jefferies B C, Wood S C, Chanock R M, Parrott R H. Epidemiology of respiratory syncytial virus infection in Washington, D.C. III. Composite analysis of eleven consecutive yearly epidemics. Am J Epidemiol. 1973;98:355–364. doi: 10.1093/oxfordjournals.aje.a121565. [DOI] [PubMed] [Google Scholar]
- 13.Brunelleschi S, Colangelo D, Bordin G, Viano I. Tachykinin receptors are present on human monocytes and play a role in rheumatoid arthritis. J Chemother. 1998;10:150–152. doi: 10.1179/joc.1998.10.2.150. [DOI] [PubMed] [Google Scholar]
- 14.Brunelleschi S, Parenti A, Ceni E, Giotti A, Fantozzi R. Enhanced responsiveness of ovalbumin-sensitized guinea-pig alveolar macrophages to tachykinins. Br J Pharm. 1992;107:964–969. doi: 10.1111/j.1476-5381.1992.tb13392.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Carolan E J, Casale T B. Effects of neuropeptides on neutrophil migration through noncellular and endothelial barriers. J Allergy Clin Immunol. 1993;92:589–598. doi: 10.1016/0091-6749(93)90083-r. [DOI] [PubMed] [Google Scholar]
- 16.Cheido M, Idova G. Neuropeptides in the immunomodulation: substance P-induced stimulation of immune response in mice. Int J Immunopharmacol. 1997;19:529–533. doi: 10.1016/s0192-0561(97)00078-7. [DOI] [PubMed] [Google Scholar]
- 17.Choi D C, Kwon O J. Neuropeptides and asthma. Curr Opin Pulm Med. 1998;4:16–24. doi: 10.1097/00063198-199801000-00004. [DOI] [PubMed] [Google Scholar]
- 18.Connors M, Giese N A, Kulkarni A B, Firestone C Y, Morse III H C, Murphy B R. Enhanced pulmonary histopathology induced by respiratory syncytial virus (RSV) challenge of formalin-inactivated RSV-immunized BALB/c mice is abrogated by depletion of interleukin-4 (IL-4) and IL-10. J Virol. 1994;68:5321–5325. doi: 10.1128/jvi.68.8.5321-5325.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Covas M J, Pinto L A, Victorino R M. Disturbed immunoregulatory properties of the neuropeptide substance P on lymphocyte proliferation in HIV infection. Clin Exp Immunol. 1994;96:384–388. doi: 10.1111/j.1365-2249.1994.tb06039.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dickerson C, Undem B, Bullock B, Winchurch R A. Neuropeptide regulation of proinflammatory cytokine responses. J Leukoc Biol. 1998;63:602–605. doi: 10.1002/jlb.63.5.602. [DOI] [PubMed] [Google Scholar]
- 21.Goetzl E J, Xia M, Ingram D A, Kishiyama J L, Kaltreider H B, Byrd P K, Ichikawa S, Sreedharan S P. Neuropeptide signaling of lymphocytes in immunological responses. Int Arch Allergy Immunol. 1995;107:202–204. doi: 10.1159/000236977. [DOI] [PubMed] [Google Scholar]
- 22.Gordon D J, Ostlere L S, Holden C A. Neuropeptide modulation of Th1 and Th2 cytokines in peripheral blood mononuclear leucocytes in atopic dermatitis and non-atopic controls. Br J Dermatol. 1997;137:921–927. [PubMed] [Google Scholar]
- 23.Graham B S, Henderson G S, Tang Y W, Lu X, Neuzil K M, Colley D G. Priming immunization determines T helper cytokine mRNA expression patterns in lungs of mice challenged with respiratory syncytial virus. J Immunol. 1993;151:2032–2040. [PubMed] [Google Scholar]
- 24.Groothuis J R, Simoes E A, Hemming V G. Respiratory syncytial virus (RSV) infection in preterm infants and the protective effects of RSV immune globulin (RSVIG). Respiratory Syncytial Virus Immune Globulin Study Group. Pediatrics. 1995;95:463–467. [PubMed] [Google Scholar]
- 25.Halliday D A, McNeil J D, Scicchitano R. A metabolite of substance P, SP7-11 is involved in the pathogenesis of inflammatory joint disease. Med Hypotheses. 1993;40:227–231. doi: 10.1016/0306-9877(93)90046-s. [DOI] [PubMed] [Google Scholar]
- 26.Henderson F W, Clyde W A, Collier A M, Denny F W, Senior R J, Sheaffer C I, Conley W G, Christian R M. The etiologic and epidemiologic spectrum of bronchiolitis in pediatric practice. J Pediatr. 1979;95:183–190. doi: 10.1016/s0022-3476(79)80647-2. [DOI] [PubMed] [Google Scholar]
- 27.Ho W Z, Lai J P, Zhu X H, Uvaydova M, Douglas S D. Human monocytes and macrophages express substance P and neurokinin-1 receptor. J Immunol. 1997;159:5654–5660. [PubMed] [Google Scholar]
- 28.Institute of Medicine. New vaccine development: establishing priorities, diseases of importance in developing countries. Washington, D.C.: National Academy Press; 1986. [PubMed] [Google Scholar]
- 29.Institute of Medicine. New vaccine development: establishing priorities, diseases of importance in the United States. Washington, D.C.: National Academy Press; 1985. [Google Scholar]
- 30.Johnson T R, Johnson J E, Roberts S R, Wertz G W, Parker R A, Graham B S. Priming with secreted glycoprotein G of respiratory syncytial virus (RSV) augments interleukin-5 production and tissue eosinophilia after RSV challenge. J Virol. 1998;72:2871–2880. doi: 10.1128/jvi.72.4.2871-2880.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Joos G F, Kips J C, Peleman R A, Pauwels R A. Tachykinin antagonists and the airways. Arch Int Pharmacodyn Ther. 1995;329:205–219. [PubMed] [Google Scholar]
- 32.Kaltreider H B, Ichikawa S, Byrd P K, Ingram D A, Kishiyama J L, Sreedharan S P, Warnock M L, Beck J M, Goetzl E J. Upregulation of neuropeptides and neuropeptide receptors in a murine model of immune inflammation in lung parenchyma. Am J Respir Cell Mol Biol. 1997;16:133–144. doi: 10.1165/ajrcmb.16.2.9032120. [DOI] [PubMed] [Google Scholar]
- 33.Karron R A, Buonagurio D A, Georgiu A F, Whitehead S S, Adamus J E, Clements-Mann M L, Harris D O, Randolph V B, Udem S A, Murphy B R, Sidhu M S. Respiratory syncytial virus (RSV) SH and G proteins are not essential for viral replication in vitro: clinical evaluation and molecular characterization of a cold-passaged, attenuated RSV subgroup B mutant. Proc Natl Acad Sci USA. 1997;94:13961–13966. doi: 10.1073/pnas.94.25.13961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Killingsworth C R, Shore S A, Alessandrini F, Dey R D, Paulauskis J D. Rat alveolar macrophages express preprotachykinin gene-I mRNA-encoding tachykinins. Am J Physiol. 1997;273:L1073–L1081. doi: 10.1152/ajplung.1997.273.5.L1073. [DOI] [PubMed] [Google Scholar]
- 35.Kim H W, Arrobio J O, Brandt C D, Jeffries B C, Pyles G, Reid J L, Chanock R M, Parrott R H. Epidemiology of respiratory syncytial virus infection in Washington, D.C. I. Importance of the virus in different respiratory tract syndromes and temporal distribution of infection. Am J Epidemiol. 1973;98:216–225. doi: 10.1093/oxfordjournals.aje.a121550. [DOI] [PubMed] [Google Scholar]
- 36.Lambert N, Lescoulie P L, Yassine-Diab B, Enault G, Mazieres B, De Preval C, Cantagrel A. Substance P enhances cytokine-induced vascular cell adhesion molecule-1 (VCAM-1) expression on cultured rheumatoid fibroblast-like synoviocytes. Clin Exp Immunol. 1998;113:269–275. doi: 10.1046/j.1365-2249.1998.00621.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lee H R, Ho W Z, Douglas S D. Substance P augments tumor necrosis factor release in human monocyte-derived macrophages. Clin Diagn Lab Immunol. 1994;1:419–423. doi: 10.1128/cdli.1.4.419-423.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lotz M, Vaughan J H, Carson D A. Effect of neuropeptides on production of inflammatory cytokines by human monocytes. Science. 1988;241:1218–1221. doi: 10.1126/science.2457950. [DOI] [PubMed] [Google Scholar]
- 39.Lucey D R, Novak J M, Polonis V R, Liu Y, Gartner S. Characterization of substance P binding to human monocytes/macrophages. Clin Diagn Lab Immunol. 1994;1:330–335. doi: 10.1128/cdli.1.3.330-335.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Luger T A, Bhardwaj R S, Grabbe S, Schwarz T. Regulation of the immune response by epidermal cytokines and neurohormones. J Dermatol Sci. 1996;13:5–10. doi: 10.1016/0923-1811(95)00485-8. [DOI] [PubMed] [Google Scholar]
- 41.Lundberg J M. Tachykinins, sensory nerves, and asthma—an overview. Can J Physiol Pharmacol. 1995;73:908–914. doi: 10.1139/y95-125. [DOI] [PubMed] [Google Scholar]
- 42.Maggi C A. The effects of tachykinins on inflammatory and immune cells. Regul Pept. 1997;70:75–90. doi: 10.1016/s0167-0115(97)00029-3. [DOI] [PubMed] [Google Scholar]
- 43.Maggi C A. The mammalian tachykinin receptors. Gen Pharmacol. 1995;26:911–944. doi: 10.1016/0306-3623(94)00292-u. [DOI] [PubMed] [Google Scholar]
- 44.Manske J M, Sullivan E L, Andersen S M. Substance P mediated stimulation of cytokine levels in cultured murine bone marrow stromal cells. Adv Exp Med Biol. 1995;383:53–64. doi: 10.1007/978-1-4615-1891-4_7. [DOI] [PubMed] [Google Scholar]
- 45.Mantyh P W. Substance P and the inflammatory and immune response. Ann N Y Acad Sci. 1991;632:263–271. doi: 10.1111/j.1749-6632.1991.tb33114.x. [DOI] [PubMed] [Google Scholar]
- 46.Martinez F D, Morgan W J, Wright A L, Holberg A L, Tausig L M. Diminished lung function as a predisposing factor for wheezing respiratory illness in patients. N Engl J Med. 1988;319:1112–1117. doi: 10.1056/NEJM198810273191702. [DOI] [PubMed] [Google Scholar]
- 47.McGillis J P, Organist M L, Payan D G. Substance P and immunoregulation. Fed Proc. 1987;46:196–199. [PubMed] [Google Scholar]
- 48.Nicolai T, Pohl A. Acute viral bronchiolitis in infancy: epidemiology and management. Lung. 1990;168(Suppl.):396–405. doi: 10.1007/BF02718157. [DOI] [PubMed] [Google Scholar]
- 49.Openshaw P J. Immunity and immunopathology to respiratory syncytial virus. The mouse model. Am J Respir Crit Care Med. 1995;152:S59–S62. doi: 10.1164/ajrccm/152.4_Pt_2.S59. [DOI] [PubMed] [Google Scholar]
- 50.Palma C, Manzini S. Substance P induces secretion of immunomodulatory cytokines by human astrocytoma cells. J Neuroimmunol. 1998;81:127–137. doi: 10.1016/s0165-5728(97)00167-7. [DOI] [PubMed] [Google Scholar]
- 51.Price J F. Acute and long-term effects of viral bronchiolitis in infancy. Lung. 1990;168(Suppl.):414–421. doi: 10.1007/BF02718159. [DOI] [PubMed] [Google Scholar]
- 52.Reynolds P N, Holmes M D, Scicchitano R. Role of tachykinins in bronchial hyper-responsiveness. Clin Exp Pharmacol Physiol. 1997;24:273–280. doi: 10.1111/j.1440-1681.1997.tb01819.x. [DOI] [PubMed] [Google Scholar]
- 53.Sparer T E, Matthews S, Hussell T, Rae A J, Garcia-Barreno B, Melero J A, Openshaw P J. Eliminating a region of respiratory syncytial virus attachment protein allows induction of protective immunity without vaccine-enhanced lung eosinophilia. J Exp Med. 1998;187:1921–1926. doi: 10.1084/jem.187.11.1921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Srikiatkhachorn A, Braciale T J. Virus-specific CD8+ T lymphocytes downregulate T helper cell type 2 cytokine secretion and pulmonary eosinophilia during experimental murine respiratory syncytial virus infection. J Exp Med. 1997;186:421–432. doi: 10.1084/jem.186.3.421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Srikiatkhachorn A, Chang W, Braciale T J. Induction of Th1- and Th2- responses by respiratory syncytial virus attachment glycoprotein is epitope and major histocompatibility complex independent. J Virol. 1999;73:6590–6597. doi: 10.1128/jvi.73.8.6590-6597.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Stokes Peebles R, Sheller J R, Johnson J E, Mitchell D B, Graham B S. Respiratory syncytial virus infection prolongs methacholine-induced airway hyperresponsiveness in ovalbumin-sensitized mice. J Med Virol. 1999;57:186–192. doi: 10.1002/(sici)1096-9071(199902)57:2<186::aid-jmv17>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- 57.Tanabe T, Otani H, Mishima K, Ogawa R, Inagaki C. Mechanisms of oxyradical production in substance P stimulated rheumatoid synovial cells. Rheumatol Int. 1996;16:159–167. doi: 10.1007/BF01419729. [DOI] [PubMed] [Google Scholar]
- 58.Tebbey P W, Hagem M, Hancock G E. Atypical pulmonary eosinophilia is mediated by a specific amino acid sequence of the attachment (G) protein of respiratory syncytial virus. J Exp Med. 1998;188:1967–1972. doi: 10.1084/jem.188.10.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Tomaki M, Ichinose M, Miura M, Hirayama Y, Yamauchi H, Nakajima N, Shirato K. Elevated substance P content in induced sputum from patients with asthma and patients with chronic bronchitis. Am J Respir Crit Care Med. 1995;151:613–617. doi: 10.1164/ajrccm.151.3.7533601. [DOI] [PubMed] [Google Scholar]
- 60.Tripp R A, Anderson L J. Cytotoxic T-lymphocyte precursor frequencies in BALB/c mice after acute respiratory syncytial virus (RSV) infection or immunization with a formalin-inactivated RSV vaccine. J Virol. 1998;72:8971–8975. doi: 10.1128/jvi.72.11.8971-8975.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Tripp R A, Moore D, Jones L, Sullender W, Winter J, Anderson L J. Respiratory syncytial virus (RSV) G and/or SH proteins alter Th1 cytokines, natural killer cells and neutrophils responding to pulmonary infection in BALB/c mice. J Virol. 1999;73:7099–7107. doi: 10.1128/jvi.73.9.7099-7107.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Waris M E, Tsou C, Erdman D D, Zaki S R, Anderson L J. Respiratory syncytial virus infection in BALB/c mice previously immunized with formalin-inactivated virus induces enhanced pulmonary inflammatory response with a predominant Th2-like cytokine pattern. J Virol. 1996;70:2852–2860. doi: 10.1128/jvi.70.5.2852-2860.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Weinstock J V, Elliott D. The substance P and somatostatin interferon-gamma immunoregulatory circuit. Ann N Y Acad Sci. 1998;840:532–539. doi: 10.1111/j.1749-6632.1998.tb09592.x. [DOI] [PubMed] [Google Scholar]
- 64.Welliver R C. RSV and chronic asthma. Lancet. 1995;346:789–790. doi: 10.1016/s0140-6736(95)91615-6. [DOI] [PubMed] [Google Scholar]
- 65.Welliver R C, Duffy L. The relationship of RSV-specific immunoglobulin E antibody responses in infancy, recurrent wheezing, and pulmonary function at age 7–8 years. Pediatr Pulmonol. 1993;15:19–27. doi: 10.1002/ppul.1950150104. [DOI] [PubMed] [Google Scholar]
- 66.Welliver R C, Gallagher M R, Ogra P L. Clinical and laboratory diagnosis of respiratory syncytial virus infection. Crit Rev Clin Lab Sci. 1981;13:213–239. doi: 10.3109/10408368109106448. [DOI] [PubMed] [Google Scholar]