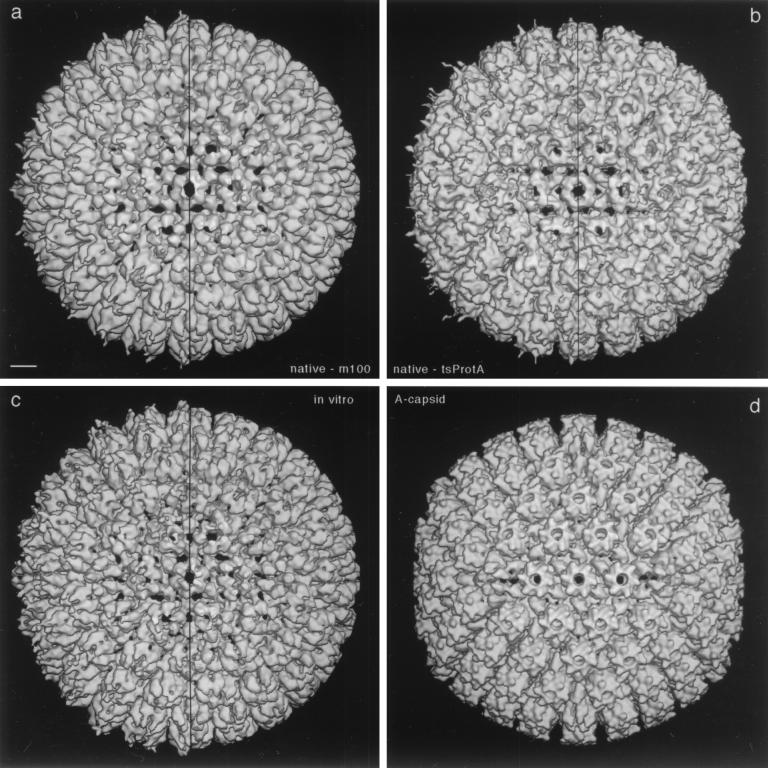
FIG. 2.
Three-dimensional reconstructions of HSV-1 procapsids from three different sources (a to c) compared to the mature capsid (d). (a) Native procapsids isolated from BHK cells infected with the mutant m100; (b) native procapsids isolated from BHK cells infected at the NPT temperature with tsProt.A; (c) procapsids assembled in vitro from lysates of Sf9 cells containing HSV-1 capsid proteins (reproduced from reference 46); (d) native A capsids isolated from BHK cells infected with wild-type HSV-1. All reconstructions are shown in surface-shaded representations as viewed along the icosahedral twofold axis of symmetry. To facilitate comparison, the reconstructions shown in panels a and d were restricted to 25-Å resolution, which is similar to the values for the reconstructions in panels b (∼28-Å resolution) and c (∼27-Å resolution). To isolate and concentrate the fragile procapsids for cryoelectron microscopy, they were precipitated with MAb 6F10 as described in Materials and Methods (24, 39). In consequence, some antibody-associated density is present around their peripheries in these density maps. The maps are represented both with this density present (left half of each panel) and with it computationally removed (right half) by setting the density to background outside a radius of ∼640 Å. Note that the three procapsids are essentially identical in structure. Bar = 100 Å.