Figure 1.
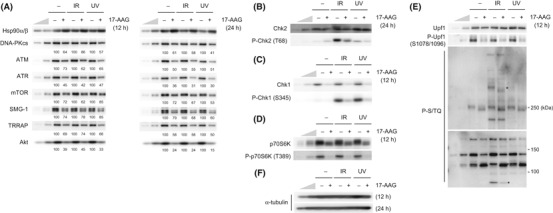
Inhibition of heat shock protein 90 (Hsp90) activity decreases the abundance of all phosphatidylinositol 3‐kinase‐related protein kinase (PIKK) proteins and the downstream signaling. (A–F) HeLa TetOff cells were treated with vehicle or 2 μM 17‐allylamino‐17‐desmethoxygeldanamycin (17‐AAG) for 12 or 24 h, then the cells were untreated, treated with 10 Gy IR, or 100 J/m2 of UV, and incubated for 1 h. Total cell lysates were analyzed by western blotting with the indicated antibodies. To estimate the protein abundance, 33 and 11% of the 17‐AAG, IR and UV‐untreated samples were loaded. The anti‐P‐S/TQ antibody recognizes phosphorylated serine or threonine in the SQ motif, potential phosphorylation sites by ATM/ATR/SMG‐1/DNA‐PKcs (E, lower panel). Asterisks indicate some examples of phosphoproteins affected by the 17‐AAG treatment (E, lower panel). ATM, ataxia telangiectasia mutated; ATR, ATM‐and Rad3‐related; DNA‐PKcs, DNA‐dependent protein kinase catalytic subunit; mTOR, mammalian target of rapamycin; SMG‐1, suppressor with morphological effect on genitalia 1; TRRAP, transformation/transcription domain‐associated protein.
