Figure 2.
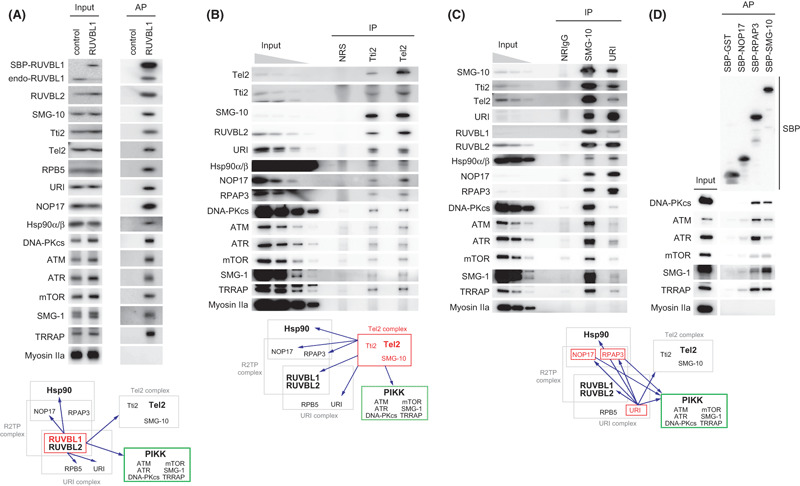
Heat shock protein 90 (Hsp90) interacts with two other phosphatidylinositol 3‐kinase‐related protein kinase (PIKK) regulators, RUVBL1/2 and Tel2 and their associated proteins. (A) RUVBL1 interacted with Hsp90, a Hsp90 co‐factor (NOP17), URI complex and Tel2 complex. Tet‐inducible streptavidin‐binding peptide (SBP)‐tagged RUVBL1 stable HEK 293 cells or control cells, which express tag peptides only, were treated with 1 ng/mL doxycycline for 3 days. Cytoplasmic cell extracts were affinity purified with streptavidin sepharose, and biotin‐eluted fractions were analyzed by western blotting with the indicated antibodies. (B,C) Protein interactions of Tel2, SMG‐10, Tti2 and URI. HeLa TetOff cells were immunoprecipitated with anti‐Tti2, anti‐Tel2 antiserum or normal rabbit serum (NRS) (B), or anti‐SMG‐10, URI, or normal rabbit IgG (NRIgG) (C). The immunoprecipitates were analyzed by western blotting with the indicated antibodies. Input: 1, 0.33, 0.11 and 0.037% (B) or 0.5, 0.17 and 0.06% (C) of the amount immunoprecipitated. (D) RPAP3 interacts with all PIKK. HeLa TetOff cells were transfected with pcDNA5/FRT/TO/NTAP‐GST, pcDNA5/FRT/TO/NTAP‐NOP17, pcDNA5/FRT/TO/NTAP‐RPAP3 or pcDNA5/FRT/TO/NTAP‐SMG‐10. The cell extracts were subjected to affinity purification with streptavidin sepharose 36 h later and analyzed by western blotting with indicated antibodies. ATM, ataxia telangiectasia mutated; ATR, ATM‐and Rad3‐related; DNA‐PKcs, DNA‐dependent protein kinase catalytic subunit; mTOR, mammalian target of rapamycin; NOP17, nucleolar protein 17; RPB5, RNA polymerase II subunit 5; SMG‐1, suppressor with morphological effect on genitalia 1; TRRAP, transformation/transcription domain‐associated protein.
