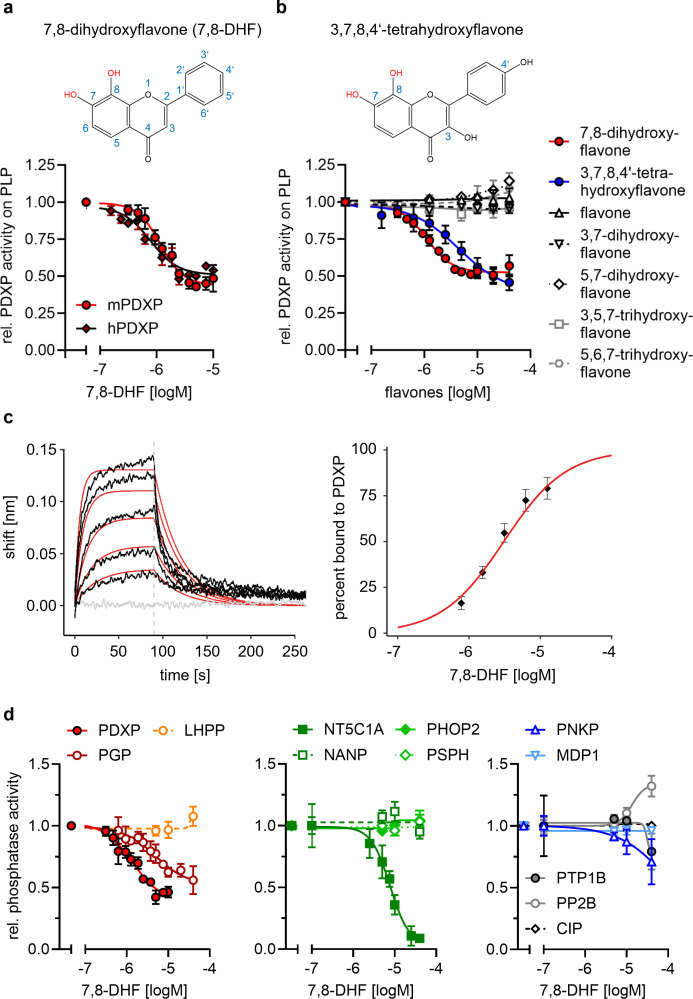
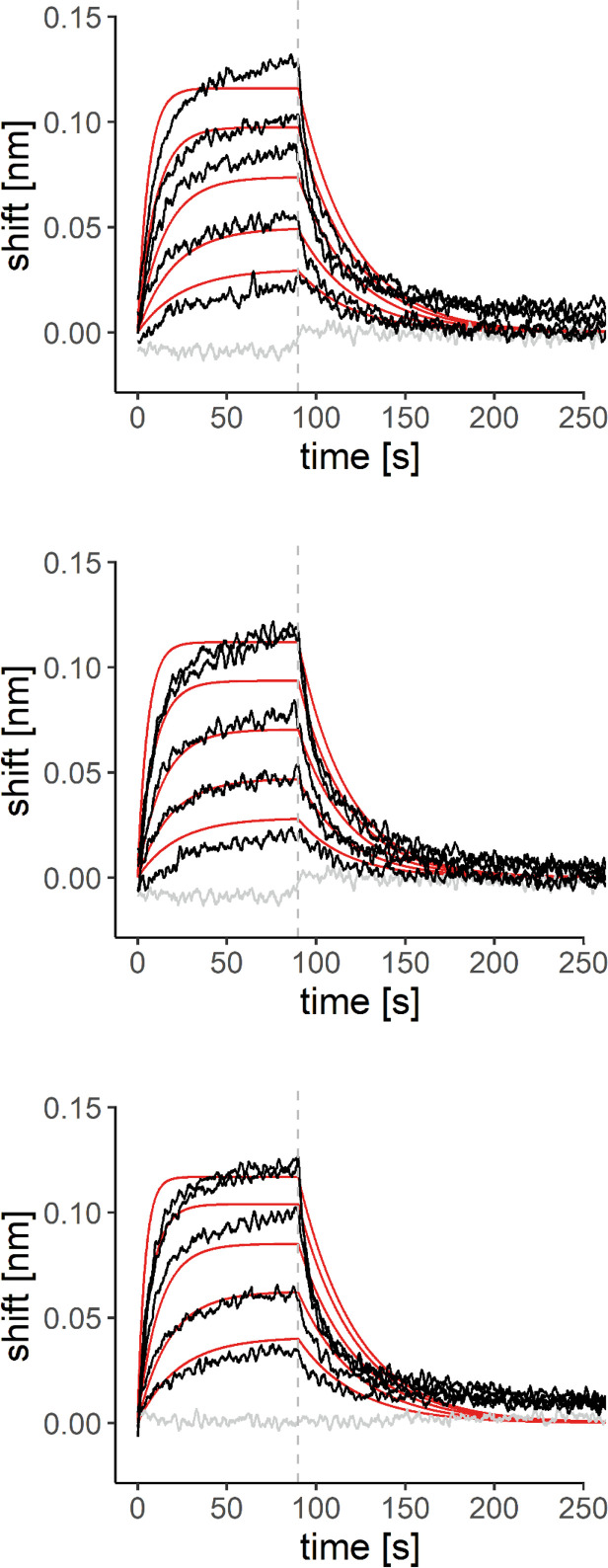
Figure 2. Characterization of the 7,8-dihydroxyflavone (7,8-DHF)/pyridoxal 5’-phosphate phosphatase (PDXP) interaction.
(a) Determination of half-maximal inhibitory constants (IC50) of 7,8-DHF (2D structure shown on top) for purified murine or human PDXP, using pyridoxal 5’-phosphate (PLP) as a substrate. Phosphatase activities in the presence of 7,8-DHF were normalized to the respective enzyme activities measured in the presence of the DMSO solvent control. Data are mean values ± SD of n=3 (human PDXP) and n=4 (murine PDXP) biologically independent experiments. (b) IC50 values of different flavones for purified murine PDXP with PLP as a substrate. Phosphatase activities in the presence of flavones were normalized to the respective enzyme activities in the presence of the DMSO solvent control. All data are mean values ± SD. The inhibition of PDXP by 3,7,8-trihydroxyflavone-4’-hydroxyphenyl (2D structure shown on top) was assessed in n=6 biologically independent experiments. All other data are from n=3 biologically independent experiments. Apparently missing error bars are hidden by the symbols. (c) Biolayer interferometry (BLI) measurements of the interaction of 7,8-DHF with purified murine PDXP. Left panel, example sensorgram overlayed with the global 1:1 binding model (red) and the negative control (gray). The dashed line indicates the start of the dissociation phase. Right panel, steady-state dose-response analysis for 7,8-DHF based on n=4 technically independent measurements. (d) Sensitivity of the indicated phosphatases to 7,8-DHF. Phosphatase activities in the presence of 7,8-DHF were normalized to the respective enzyme activities measured in the presence of the DMSO solvent control. Data are mean values ± SD of n=4 (PGP) or n=3 biologically independent experiments (all other phosphatases). Phosphatase substrates and haloacid dehalogenase (HAD) phosphatase cap types are indicated in parentheses. PDXP, pyridoxal 5’-phosphate phosphatase (pyridoxal 5’-phosphate, C2); PGP, phosphoglycolate phosphatase (2-phosphoglycolate; C2); LHPP, phospholysine phosphohistidine inorganic pyrophosphate phosphatase (imidodiphosphate; C2); NT5C1A, soluble cytosolic 5'-nucleotidase 1A (AMP; C1); NANP, N-acetylneuraminate 9-phosphate phosphatase (6-phosphogluconate; C1); PHOP2, phosphatase orphan 2 (pyridoxal 5’-phosphate; C1); PSPH, phosphoserine phosphatase (O-phospho-L-serine; C1); PNKP, polynucleotide kinase phosphatase (3-phospho-oligonucleotide; C0); MDP1, magnesium-dependent phosphatase-1 (D-ribose-5-phosphate; C0); PTP1B (protein tyrosine phosphatase 1B; EGFR phospho-peptide); PP2B, protein phosphatase 2B/calcineurin (PKA regulatory subunit type II phospho-peptide); CIP, calf intestinal phosphatase (pNPP). Source data are available for this figure.