Summary
Magnaporthe oryzae secretes several effectors that modulate and hijack rice processes to colonize host cells, but the underlying mechanisms remain unclear. We report on a novel cytoplasmic effector MoIug4 that targets the rice ethylene pathway as a transcription repressor to subvert host immunity.
We found that MoIug4 binds to the promoter of the host OsEIN2 gene that encodes a central signal transducer in the ethylene-signaling pathway. We also identified a MoIug4 interacting protein, OsAHL1, which acts as an AT-hook motif-containing protein binding to the A/T-rich promoter regions. Our knockout and overexpression studies showed that OsAHL1 positively regulates plant immunity in response to M. oryzae infection.
OsAHL1 exhibits transcriptional regulatory activities by binding the OsEIN2 promoter region, similar to MoIug4. Intriguingly, we found that MoIug4 exhibits a higher binding affinity than OsAHL1 to the OsEIN2 promoter, suggesting differential regulatory specificities.
These results revealed a counter-defense strategy by which the pathogen effector suppresses the activation of host defense genes by interfering with host transcription activator functions.
Keywords: effector, ethylene signaling, Magnaporthe oryzae, plant immunity, transcription reprogramming
Introduction
Plants are constantly challenged by various pathogens, including bacteria, viruses and fungi (Jones & Dangl, 2006; Boller & He, 2009; Zhou & Zhang, 2020). Plants have a two-tier innate immune system: recognition of pathogen-associated molecular patterns (PAMPs) activates the mitogen-activated protein kinase (MAPK)-signaling pathway resulting in transcriptional reprogramming of defense-related genes (Ausubel, 2005). Conversely, the pathogens secrete a multitude of effector proteins into host cells or apoplast spaces to subvert plant immunity. Following pathogen-secreted effectors were specifically recognized by resistant proteins, effector-triggered immunity (ETI) is activated, leading to fiercer, more robust responses. Identification of effectors and uncovering their functions provide critical insights into microbial pathogenesis mechanisms and reveal regulatory functions of innate immunity in planta (Boller & He, 2009; Giraldo & Valent, 2013).
The hemibiotrophic fungal pathogen Magnaporthe oryzae and the monocotyledonous host plant rice (Oryza sativa L.) serve as an important pathogen–host interaction and effector function model (Wilson & Talbot, 2009; Zhang et al., 2016). The rice blast also remains a significant crop disease of significant economic importance. Defense against the blast is characterized by the accumulation of reactive oxygen species (ROS) and the deposition of callose that are both PAMP-triggered immunity (PTI) responses (Park et al., 2012, 2016; Giraldo & Valent, 2013; Liu & Zhang, 2021). These responses accompany the biosynthesis of signal molecules to induce host immunity, such as programmed cell death (Chi et al., 2009).
Encountering various abiotic and biotic environmental stresses, plants have developed sophisticated mechanisms to sense the environmental conditions and modulate their growth and development patterns to cope with or adapt to different stress conditions. Previous studies showed that plant hormones such as SA, JAs and ethylene act as signals to trigger and mediate diverse defense responses (Spoel & Dong, 2008), and hormones auxin, gibberellic acids, brassinosteroids and ABA also are actively involved in regulating normal growth, development and immunity in plants (Bari & Jones, 2009; Yang et al., 2013; Helliwell et al., 2016).
Magnaporthe oryzae was demonstrated to deliver various effector molecules into rice cells. For example, the secreted LysM protein 1 (MoSlp1) and MoAa91 were shown to compete with the host OsCEBiP protein in chitin binding, thereby preventing PTI activation (Mentlak et al., 2012; Chen et al., 2014; Li et al., 2020). Recently, the rice tetratricopeptide repeat protein OsTPR1 was shown to interact with MoChia1 in the rice apoplast, whose competitively binding by MoChia1 allows the accumulation of free chitin and the reestablishment of immune response (Yang et al., 2019b). AvrPiz-t, a key effector, modulates rice immunity through functions in suppressing the ubiquitin ligase activity of the rice RING E3 ubiquitin ligase APIP6, the degradation of E3 ligase, and the AvrPiz-t interacting protein 5 (APIP5) transcriptional activity (Park et al., 2012, 2016; Wang et al., 2016). A recent study also showed that two M. oryzae nuclear effectors modulate host immunity through transcription reprogramming (Kim et al., 2020). Other studies showed that M. oryzae represses host immunity by impairing hormone-based defense signaling pathways. Following invading cells, the fungus secretes an antibiotic biosynthesis-related monooxygenase into the host that can alter host JA into a hydroxylated form (12OH-JA). 12OH-JA disrupts the JA-dependent defense network in the host, facilitating fungal colonization (Patkar et al., 2015). Magnaporthe oryzae also synthesizes its own ABA and cytokinins and then uses them as virulence factors to attenuate host immune responses (Spence et al., 2015; Chanclud et al., 2016). Despite such findings, additional mechanisms by which M. oryzae effectors subvert rice immunity remain to be explored.
Magnaporthe oryzae isolate-specific genes are important factors for strain-specific virulence and provide a putative pool for effector identification. Previous studies predicted 134 candidate effectors from M. oryzae, including MoIug6 and MoIug9, which play important roles in suppressing hormone-mediated defense responses and pathogenicity (Dong et al., 2015). We here show that MoIug4 is a zinc-finger protein required for full virulence. Using chromatin immunoprecipitation (ChIP) and electrophoretic mobility shift assay (EMSA) analysis, we demonstrated that MoIug4 effectively suppresses the expression of OsEIN2 by binding to its promoter. In addition, we showed that MoIug4 exhibits a higher affinity than OsAHL1, a DNA-binding protein regulating rice resistance to M. oryzae, in binding to the OsEIN2 promoter and affecting its transcription. Our study reveals a novel regulatory mechanism evolved in a plant pathogen that modulates the host defense response transcriptionally.
Materials and Methods
Culture conditions and plant infection assays
All strains were cultured on complete medium (CM) agar plates at 28°C (Liu et al., 2018). The M. oryzae isolate Guy11 was used as the wild-type (WT) strain in this study. Protoplasts were prepared and transformed as described previously (Guo et al., 2011; Liu et al., 2019). Mycelia were harvested from liquid CM for genomic DNA and RNA extraction. The radial growth was measured after incubation for 7 d and then photographed. All experiments were repeated three times, each with three replicates.
Resistance test and infection assays
For resistance tests, conidia were suspended to a concentration of 5 × 104 spores ml−1 in 0.2% (w/v) gelatin solution. Five milliliters of suspension was sprayed on 2-wk-old rice seedlings. Inoculated plants were kept in a growth chamber at 25°C with 90% humidity and in the dark for the first 24 h, followed by a light : dark cycle (12 h : 12 h). The disease severity was assessed at 7 d post-inoculation (dpi) (Zhang et al., 2011; Liu et al., 2019). To observe the penetration and invasive growth in rice cells, conidial suspensions (1 × 105 spores ml−1) were injected into the leaf sheath. At 28°C for 24 h, the inner epidermis of infected sheaths was observed under a microscope (Yin et al., 2019a).
Gene disruption and complementation
In order to generate the MoIUG4 gene replacement vector pCX62, c. 1-kb upstream and 1-kb downstream fragments were cloned. The resulting PCR products were ligated to the hygromycin resistance cassette released from pCX62, as described previously (Dong et al., 2015). Putative mutants were screened by PCR and confirmed by Southern blotting analysis. To complement the ΔMoiug4 mutant strain, the DNA fragment, including the putative promoter and the coding sequence, was amplified and inserted into pYF11 (bleomycin resistance). The plasmids were extracted and transformed into Escherichia coli competent cells, and then the plasmids with correct inserts were transferred into protoplasts, as described previously (Li et al., 2017). To observe the secretion in rice cells, the coding sequences of MoIUG4 with their native promoters were fused with green fluorescent protein (GFP) in pYF11.
RNA isolation, quantitative reverse transcription and quantitative real-time (qRT)-PCR
RNA isolation was performed by TRIzol (Invitrogen). cDNA synthesis was performed by PrimeScript™ RT Reagent Kit (TaKaRa, Dalian, China). The qRT-PCR was performed with the ABI 7500 Fast Real-Time System (Thermofisher, Waltham, MA, USA), and transcripts were analyzed by the 7500 System sds software. To compare the relative abundance of the target gene transcripts in different rice varieties, the average threshold cycle (Ct) was normalized to the rice actin for each treated samples as , where . Fold-changes during different rice varieties were calculated as , where .
Chromatin immunoprecipitation-Seq analyses
Chromatin immunoprecipitation was performed according to the described protocol with additional modifications. MoIUG4-OX lines of rice were cultured for 3 wk. Briefly, the leaves of MoIUG4-OX lines were cross-linked with 1% formaldehyde for 15 min and then stopped with 125 mM glycine. The cultures were ground with liquid nitrogen and resuspended in lysis buffer (250 mM, HEPES pH 7.5, 150 mM NaCl, 1 mM EDTA, 1% Triton, 0.1% Deoxy Cholate, 10 mM DTT) and protease inhibitor (Sangon Co., Shanghai, China). DNA was sheared into c. 500-bp fragments with 20 pulses of 10 and 20 s of resting at 35% amplitude (Qsonica*sonicator, Q125; Branson, MO, USA). After centrifugation, the supernatant was diluted with 10× chromatin immunoprecipitation (ChIP) dilution buffer (1.1% Triton X-100, 1.2 mM EDTA, 16.7 mM Tris–HCl, pH 8.0 and 167 mM NaCl). Immunoprecipitation was performed using monoclonal anti-GFP ab290 (Abcam, Cambridge, UK; 1 : 500 dilution) antibody. After washing serially with low-salt wash buffer (one time; 150 mM NaCl, 20 mM Tris-HCl (PH 8.0), 0.2% SDS, 0.5% TritonX-100, 2 mM EDTA), high-salt wash buffer (one time; 500 mM NaCl, 20 mM Tris-HCl (PH 8.0), 0.2% SDS, 0.5% TritonX-100, 2 mM EDTA), LiCl wash buffer and TE buffer (two times; 100 mM Tris-HCl (PH 8.0), 10 mM EDTA), DNA was eluted from beads with elution buffer (1% SDS, 0.1 M NaHCO3). DNA then was immunoprecipitated by ethanol followed by washing, eluting, reversing the cross-linking and digesting with proteinase K. Further, ChIP-enriched DNA was sequenced on an Illumina HiSeq2500 (RuiYuan Biotechnology and Novogene, Nanjing, China). The accession number for RNA-seq data reported in this paper is GSE188802 in GEO datasets (http://www.ncbi.nlm.nih.gov/gds).
In vitro pull-down assay
In order to construct glutathione S-transferase (GST)-fusion plasmids, OsAHL1, OsAHL1NT and OsAHL1CT were inserted into the vector pGEX4T-2, respectively (GE Healthcare Life Science, Marlborough, MA, USA). To construct the His-fusion plasmid, MoIUG4 was inserted into the vector pET-32a (Navogen, Las Vegas, NV, USA). The pull-down assay was carried out using a ProFound pull-down GST protein-protein interaction kit (Pierce, Waltham, MA, USA) according to the manufacturer’s instructions. Briefly, GST, OsAHL1-GST, OsAHL1NT-GST or OsAHL1CT-GST was expressed in E. coli strain BL21 (DE3). Soluble proteins were incubated with 50 μl glutathione agarose beads (Invitrogen) for 2 h at 4°C. The beads were washed five times and then incubated with equal amounts of bacterial lysates containing MoIug4-His for another 2 h at 4°C. The beads were washed five times again, and the presence of MoIug4-His was detected by immunoblotting using the anti-His antibody (Abmart Inc., Berkeley Heights, NJ, USA) (Yin et al., 2019b).
Bimolecular fluorescence complementation (BiFC) assay
The OsAHL1-cYFP, OsAHL1NT-cYFP, OsAHL1CT-cYFP fusion construct was generated by cloning OsAHL1 fragments into pHZ68 (BiFC vectors from J. R. Xu, Purdue University) (YFP, yellow fluorescent protein). Likewise, the MoIug4-nYFP fusion construct was generated into pHZ65 (BiFC vectors from J. R. Xu, Purdue University). Construct pairs of OsAHL1-cYFP and MoIug4-nYFP, OsAHL1NT-cYFP and MoIug4-nYFP, and OsAHL1CT-cYFP and MoIug4-nYFP were co-introduced into rice protoplasts, respectively. YFP signals were examined with a Zeiss LSM710 confocal microscope.
Yeast two-hybrid (Y2H) assay
The bait constructs were generated by cloning full-length cDNA (OsAHL1, OsAHL1NT and OsAHL1CT) into pGADT7 (LM1010; LMAI Biotechnology, Shanghai, China). The CDS region of MoIug4 without signal peptide was cloned into pGBKT7 as prey constructs. BD library was generated by TaKaRa. The resulting prey and bait constructs were confirmed by sequencing analysis and transformed in pairs into yeast strain AH109 (LM1010; LMAI Biotechnology) following the manufacturer’s recommended protocol (BD Biosciences Clontech, Franklin Lake, NJ, USA). Transformants grown on synthetic dextrose medium lacking leucine and tryptophan (SD-Leu-Trp) were transferred to synthetic medium lacking leucine, tryptophan and histidine (SD-Leu-Trp-His). Yeast strains for positive and negative controls were provided by the BD library construction & screening kit.
Co-immunoprecipitation (co-IP)
In order to confirm in vivo interactions, the MoIug4-GFP and OsAHL1-FLAG fusion constructs were prepared. Then different pairs of specific constructs were co-transformed into protoplasts of the rice. Our protoplast isolation protocol was based on the protocol for protoplasts provided online by J. Sheen’s laboratory (http://genetics.mgh.harvard.edu/sheenweb) with several changes. Rice seeds were grown as stated above. Between 7- and 14-d post-germination, plants were c. 10–20 cm tall. Leaf and stem tissue were cut into 0.5-mm pieces using very sharp razors. Tissue was immediately incubated in enzyme solution (0.6 M mannitol, 10 mM MES (pH 5.7), 1.5% Cellulase RS, 0.75% Macerozyme, 0.1% BSA, 1 mM CaCl2, 5 mM β-mercaptothion and 50 μg ml−1 carbenicillin) for 4 h in the dark under gentle shaking (40 rpm). After incubation, protoplasts were passed through a 35-μm nylon mesh filter. One volume of W5 solution (154 mM NaCl, 125 mM CaCl2, 5 mM KCl, 2 mM MES (pH 5.7)) was added and the solution was centrifuged for 5 min at 400 g to pellet the protoplasts. Cells were re-suspended in MMG solution (0.6 M mannitol, 15 mM MgCl2, 4 mM MES (pH 5.7)) for polyethylene glycol (PEG)-mediated transformation at 106 cells ml−1. Cells were quantified using a hemocytometer. For transformation, 40% PEG (0.6 M mannitol, 100 mM CaCl2, 40% v/v PEG 3350) was added to the protoplasts for 15 min. Cells were washed with 10 volumes of W5 and then re-suspended in incubation solution (0.6 M mannitol, 4 mM MES (pH 5.7), 4 mM KCl). Cells were incubated at 28°C in the dark overnight. Then, the total proteins were isolated from different positive transformants and incubated with anti-GFP agarose (gta-20; Chromo Tek, Martinsried-Planegg, Germany) at 4°C for 2 h with gentle shaking. Proteins bound to the beads were eluted after a series of washing steps by 1× PBS (diluted from 10× PBS (ST476; Beyotime Biotechnology, Shanghai, China)). Elution buffer (200 mM glycine, pH 2.5) and neutralization buffer (1 M Tris base, pH 10.4) were used for the elution process. Total, suspension and eluted proteins then were analyzed by Western blot analysis using anti-GFP (mouse, 1 : 5000, 293967; Abmart) or anti-FLAG (rabbit, 1 : 5000, M20018; Abmart) antibodies.
Ethylene quantification
Ethylene (ET) accumulation was measured according to the method of Kim et al. (2016). Gas samples (1 ml) were injected into a gas chromatograph to quantify ET gas concentrations using a gas-tight syringe. The gas chromatograph (Agilent 6850; Agilent Technologies, La Jolla, CA, USA) was equipped with an HP-PLOT U column (0.32 mm, 10 ml) and a hydrogen flame ionization detector. The injection temperature was 120°C, and the detector temperature was 220°C. The column was held for 8 min at 50°C. The ET production was assessed by measuring the peak area on the chromatogram relative to the standard curve. For ET measurements during M. oryzae infection, rice leaves were incubated with 4 ml conidia suspension of the indicated strains (1 × 105 spores ml−1) after measuring the FW. The leaves then were sealed in gas chromatography vials and incubated at 48 h post-inoculation (hpi). The ET accumulation then was measured using gas chromatography as described above; 10% ET gas was used as a control to construct a standard curve in each experiment.
Electrophoretic mobility shift assay (EMSA) and luciferase reporter analyses
For the EMSA assay, the DNA fragment from the OsEIN2 promoter was end-labeled with Alex660 by PCR amplification using the Alex660-labeled primer. The purified protein was mixed with Alex660-labeled DNA, incubated for 20 min at 25°C in binding buffer (GS009-1; Co. Beyotime Biotechnology), and separated by agarose gel electrophoresis. Gels were visualized directly using an Odyssey scanner (Li-Cor, Lincoln, NE, USA) with excitation at 700 nm (Qian et al., 2022). For the transcriptional activation assay, the promoter fragment of OsEIN2 was inserted into reporter plasmid pICSL86900, which contained a Renilla luciferase (LUC) gene under the control of the CaMV 35S promoter used as the positive control. EIN2-LUC was used as the negative control. The effector and target plasmids and the reporter plasmids were co-transformed into Nicotiana benthamiana by Agrobacterium tumefaciens-mediated transient gene expression assays. After culturing for 24 hpi in N. benthamiana, the activities of LUC were separately determined using a Dual-Luciferase Reporter Assay System (Promega; E1910).
Microscale thermophoresis assay (MST)
The binding of the OsAHL1-GST fusion protein to the OsEIN2 operon promoter was determined by MST using Monolith NT.115 (NanoTemper Technologies GmbH, Munich, Germany), according to the protocol described recently (Mittler et al., 2011). A constant concentration (10 M) of the labeled OsEIN2 promoter in the MST buffer (50 mM Tris, pH 7.5, 150 mM NaCl, 10 mM MgCl2, 0.05% Tween 20) was titrated against increasing concentrations of OsAHL1-GST and MoIug4-HIS dissolved in PBST. Both OsAHL1-GST and MoIug4-HIS protein were labeled with the fluorescent dye NT-647-NHS (NanoTemper Technologies) via amine conjugation. A constant concentration (1 μM) of the labeled promoter in the MST buffer was titrated against OsAHL1-GST (concentration range 3.0–20 mM). MST premium-coated capillaries (Monolith NT.115 MO-K005) were used to load the samples into the MST instrument using 40% MST power and 40% LED power. The binding of OsAHL1-GST and MoIug4-HIS was detected by this system with similar test conditions. The pairs containing the deletion of the cis-element of OsEIN2 were used as the negative control. All experiments were performed in triplicate. Data were analyzed using Nanotemper Analysis v.1.2.101 software (NanoTemper Technologies).
Statistical analysis
Each experiment was performed with at least three replicated measurements and represented as the mean ± standard deviation (SD). The significant differences between treatments were statistically determined by one-way ANOVA comparison and followed by a Student’s t-test if ANOVA analysis was significant at P < 0.05 or P < 0.01.
Results
Identification of MoIug4 as a novel effector protein and localization in planta
The M. oryzae genome contains at least 134 additional genes encoding putative effectors. After screening 134 mutant strains of candidate effector genes by gene-knockout, we focused on MoIug4 containing a C-terminal zinc-binding domain (Dong et al., 2015). We found that MoIUG4 encoding MoIug4 is a single copy gene in Guy11 (Supporting Information Table S1) and its expression is linked to infection as higher expression levels were detected 24–36 hpi (Fig. 1a). To further evaluate the role of MoIug4, we generated a gene knockout mutant by replacing the MoIUG4 gene with the hygromycin-resistance gene (HPH) cassette in Guy11 (Fig. S1). To examine the virulence impact of MoLUG4 mutation, conidial suspensions (5 × 104 spores ml−1) of the ΔMoiug4 mutant, Guy11 and the complemented mutant strain were sprayed onto 2-wk-old rice seedlings (cv. CO-39). Despite very few typical lesions, the ΔMoiug4 mutant caused fewer and restricted lesions on rice leaves 7 dpi, in contrast to numerous characteristic lesions in control (Fig. 1b). The lesions were quantitatively examined by a ‘lesion type’ scoring assay (Wang et al., 2013), and the results showed that the ΔMoiug4 mutant produced mostly restricted lesions (Fig. 1c). In addition, conidial suspensions (1 × 105 spores ml−1) were infiltrated into the sheath of 4-wk-old rice seedlings. The ΔMoiug4 mutant caused fewer and more restricted lesions on the rice leaves than the control (Fig. 1d). These results demonstrated that MoIug4 is required for full virulence of M. oryzae.
Fig. 1.
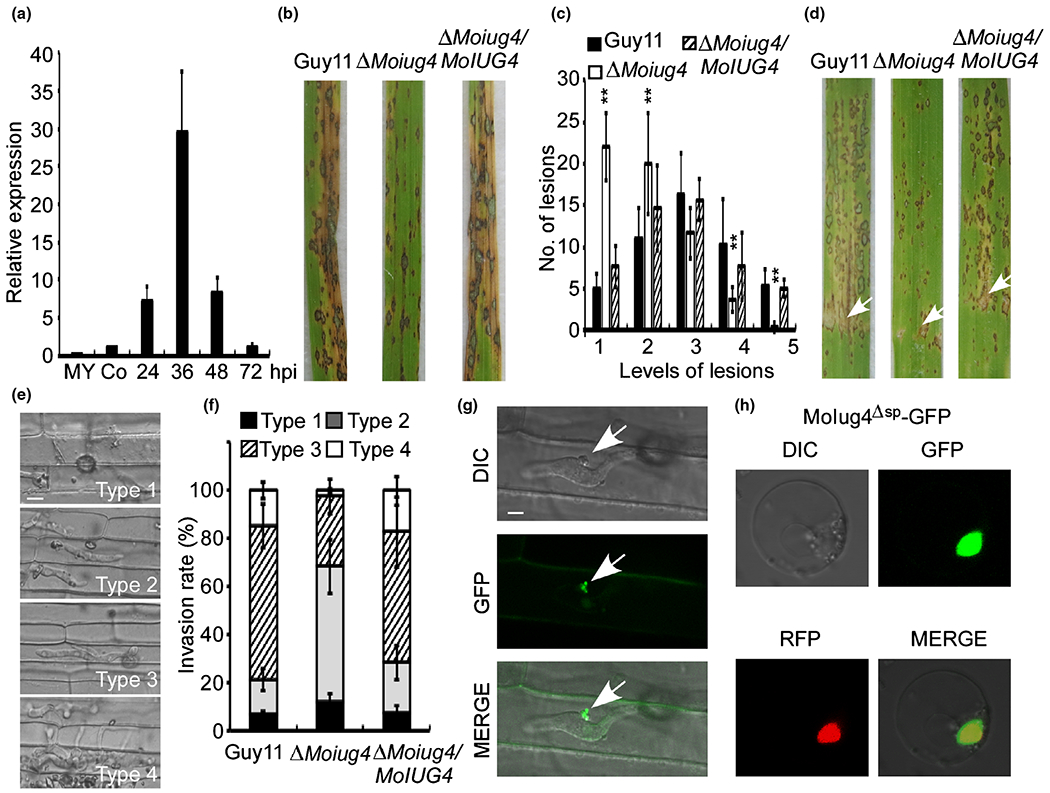
MoIug4 functions as a cytoplasm effector affecting the virulence of Magnaporthe oryzae. (a) Expression of MoIUG4 during the infection by M. oryzae. The transcription level of MoIUG4 at different stages of infection was determined by quantitative real-time (qRT)-PCR. Two-week-old leaves from cv. CO-39 were inoculated with conidia suspensions of M. oryzae. Total RNA was extracted from mycelia, conidia and infected leaves at 24, 36, 48 and 72 h post-inoculation (hpi). Error bars indicate SD of three biological replicates. (b) Pathogenicity assay on rice leaves. Conidial suspensions of strains were sprayed onto 2-wk-old rice seedlings (CO-39). Diseased leaves were photographed 7 d post-inoculation (dpi). (c) Quantification of lesion type. Lesions were photographed and measured at 7 dpi, counted within an area of 4 cm2, and experiments were repeated three times with similar results. The asterisks indicate a significant difference according to Student’s t-test (P < 0.01). (d) Leaves from 4-wk-old rice seedlings were injected with conidial suspension (5 × 104 spores ml−1) of Guy11, the ΔMoiug4 mutant and the complemented strain. Diseased leaves were photographed 7 dpi. White arrows indicate the injected sites. (e) Excised rice sheath cells from 3-wk-old rice seedlings were inoculated with conidial suspensions (1 × 105 spores ml−1). Infectious growth was observed at 36 hpi. We grouped the invasive hyphae (IH) into four types (1, only appressoria; 2, only a single invasive hypha with no branch; 3, IH extended but was limited in one plant cell; 4, IH with numerous branches and extended to surrounding cells) for further elaborate observations. Bar, 5 μm. (f) Statistical analysis for each type of infectious hyphal shape, for each tested strain, > 100 infecting hyphae were counted per replicate, and the experiment was repeated three times. (g) Localization of full-length MoIug4 was observed in the rice sheath cells during infection. White arrows, biotrophic interfacial complex (BIC). Bar, 5 μm. (h) Localization of MoIug4 in the rice protoplast cells. MoIug4Δsp-GFP was transformed into the rice protoplast and observed under the eGFP channel; H1-RFP was used as the nuclear marker (GFP and RFP, green and red fluorescent proteins).
In order to further examine the virulence attenuation in the ΔMoiug4 mutant, we performed an excised leaf sheath assay to test the appressorial penetration rates and infectious hyphae (IH) extension ability within the host cell. After incubation with spore suspension (1 × 105 spores ml−1) for 36 h, most of the appressoria from the ΔMoiug4 mutant penetrated the rice tissue, but IH were mostly types 2 and 3, in contrast to mostly types 3 and 4 in Guy11 (Fig. 1e,f). At 36 hpi, ΔMoiug4 type 2 and type 3 IH were still limited within one plant cell, whereas Guy11 type 3 and type 4 IH showed free spreading to adjacent cells (Fig. 1e,f). These results indicated that MoIug4 is important for the pathogenicity of M. oryzae and that virulence attenuation was due to a defect in IH expansion.
Previous studies indicated the effectors are secreted and accumulated at the biotrophic interfacial complex (BIC) or within the extra-invasive hyphal membrane (EIHM) (Giraldo et al., 2013). To study MoIug4 localization in the host, we expressed the MoIUG4:GFP fusion gene under the control of its native promoters in Guy11 and observed GFP fluorescence expression in the invaded rice sheath cells at 24 hpi by confocal fluorescence microscopy (Zeiss LSM710, ×63 oil). Fluorescence was detected in BIC (Fig. 1g), indicating that MoIug4 could be delivered into the rice cell. We also examined the secretion of MoIug4 using the MoIug4-NLS (nuclear localization signaling) construct and found fluorescence accumulation occurs in the rice nuclei (Fig. S2). Further, we introduced pBIN-MoIUG4Δsp into the rice protoplast, and N. benthamiana leaves. H1-RFP and DAPI colocalization confirmed that MoIug4 is localized in the nucleus (Figs 1h, S3).
MoIug4 is important for host-derived immunity and identification of MoIug4 binding targets in planta
Pathogen-associated molecular pattern (PAMP)-trigged reactive oxygen species (ROS) accumulation is a broad strategy of how a host plant defends against pathogen invasion. To explore the mechanism of virulence attention in the ΔMoiug4 mutant, we examined host-derived ROS concentrations by staining with 3, 3′-diaminobenzidine (DAB) at 24 hpi. Guy11 IH-infected host cells were not stained by DAB, as no reddish-brown precipitate was seen. By contrast, cells penetrated by ΔMoiug4 IH showed intense reddish-brown around the appressoria and infected cells (58.63 ± 6.72%), suggesting heavy stain by DAB (Fig. 2a). This indicated that deletion of MoIUG4 resulted in ROS accumulation near the infected sites. Moreover, by using diphenyleneiodonium (DPI) that inhibits plant NADPH oxidase activities (Bolwell et al., 1998; Grant et al., 2000) and Aspergillus niger catalase (CAG; Sigma), the infection defect of the ΔMoiug4 mutant was suppressed (Fig. 2a,b). This observation suggested that the scavenging of host-derived ROS at the infection site is important for the virulence of the blast fungus.
Fig. 2.
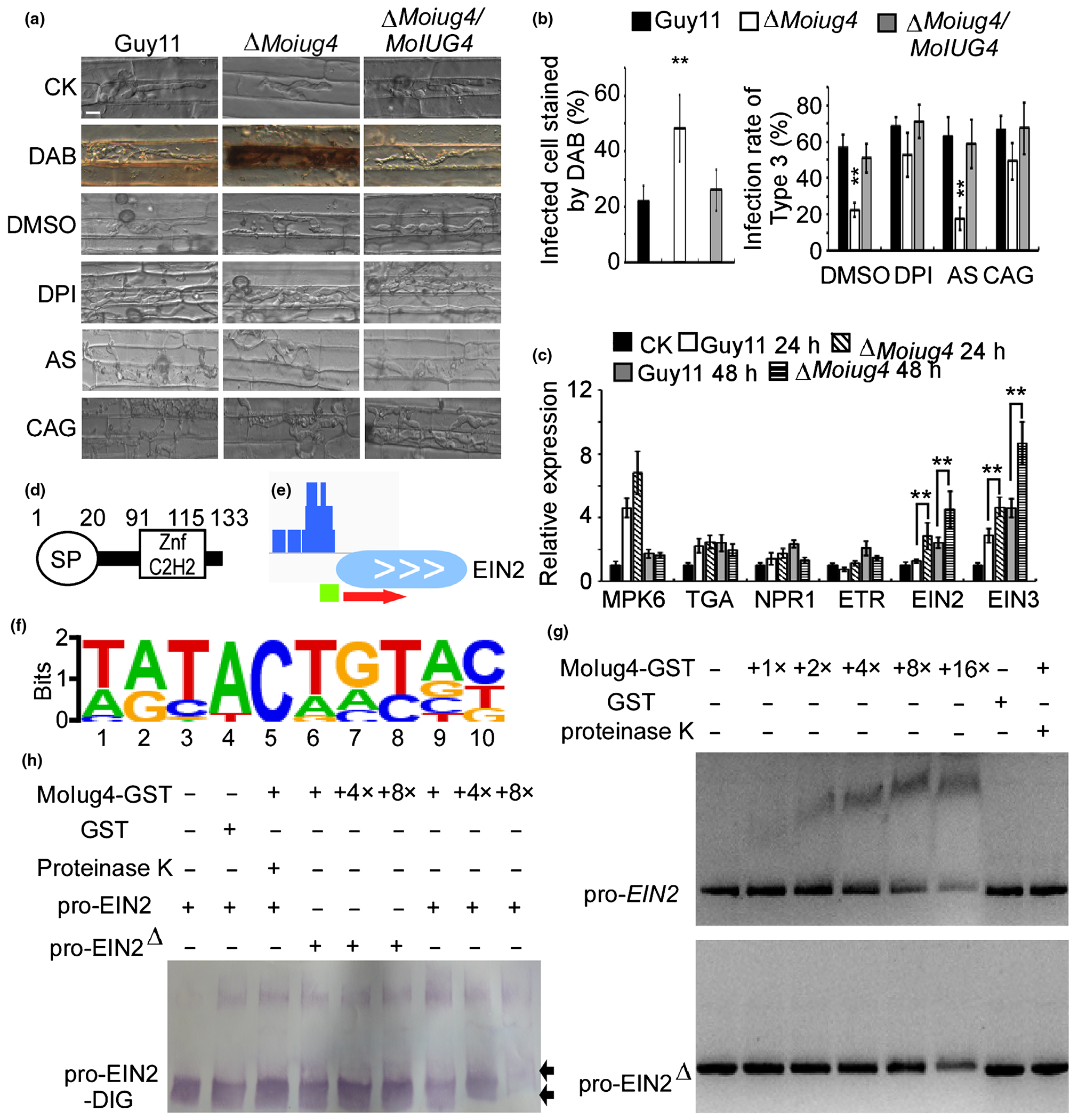
Magnaporthe oryzae MoIug4 binds with the promoter of Oryza sativa (Os)EIN2. (a) DAB staining of the excised leaf sheath of rice infected by Guy11, the ΔMoiug4 mutant, and the complemented strain at 24 h post-inoculation (hpi). The excised sheath of rice was inoculated with conidial suspensions after being treated with or without 0.5 μM DPI dissolved in DMSO. Three independent experiments were performed. The rice sheath also was inoculated with conidial suspensions after being treated with or without 0.2 U catalase (CAG) dissolved in 10 mM (NH4)2SO4 (AS). Samples were harvested and observed at 36 hpi. This experiment was performed with three independent repeats, and representative results from one of these experiments were presented. Bar, 5 μm. (b) Quantification of infected cells stained by DAB. Infectious hyphae (IH) treated with DPI and CAG were analyzed; > 50 infected rice cells were calculated with three replicates each time. Error bars represent SD, asterisks indicate a significant difference according to Student’s t-test (P < 0.01). (c) The transcription of OsMPK6, OsTGA, OsNPR1, OsETR, OsEIN2 and OsEIN3 in the infected rice was assayed using quantitative real-time (qRT)-PCR. RNA samples were collected from rice plants at 24 hpi with the ΔMoiug4 mutant or Guy11 strain. The average threshold cycle of triplicate reactions was normalized by the stable expressions of the gene elongation factor 1α (Os03g08020) in rice. Three independent biological experiments were performed that yielded similar results. Error bars represent SD, asterisks indicate a significant difference according to Student’s t-test (P < 0.01) (d) Domain architectures of MoIug4. (e) Chromation immunoprecipitation (ChIP)-Seq assays showed that MoIug4 binds to the promoter OsEIN2. The output-DNA sample was visualized in blue. The red arrow indicates the gene direction from 5′ to 3′. The green bar represents the gene promoter region, covered during ChIP-Seq. (f) The cis-element of MoIug4 was predicted by Meme. The putative sequence of the cis-element was obtained by analyzing the promoters of MoIug4 binding genes with the Meme program. (g) The full-length promoter and also the cis-element mutation promoter (EIN2Δ, including the element TGTACACAG in the promoter of OsEIN2) of OsEIN2 was incubated without (leftmost lane) or with (second to the sixth lane with increasing amounts of MoIug4) purified MoIug4 and GST proteins (the seventh lane). The proteinase K was added after the incubation of MoIug4 with DNA (rightmost lane). DNA-protein complexes were separated by electrophoresis on a 1.2% agarose gel and stained by ethidium bromide. (h) The DIG-labeled promoter of OsEIN2 was incubated with an increasing amount of MoIug4 before separation by PAGE. pro-OsEIN2: full-length promoter. pro-OsEIN2Δ: the cis-element deletion promoter. Black arrow, migrated DNA.
The ΔMoiug4 mutant also failed to suppress the defense responses and the expression of SA- and JA-related genes (TGA, NPR1 and MPK6) was found to be similar in Guy11 and the ΔMoiug4 mutant. By contrast, the expression of OsEIN2 and OsEIN3, the core regulatory genes in the ET defense gene pathway, were induced upon ΔMoiug4 infection (Fig. 2c). These findings suggested that MoIug4 does not affect the SA and JA pathways, but rather the ET pathway. We next measured ET production in rice after infection with the ΔMoiug4 mutant at 48 hpi, ET production was significantly induced compared to that infected by Guy11 (Fig. S4a). We also evaluated the expression of several ethylene synthase genes, including OsACO2, OsACS2 and OsEFR2, and found induced expression of OsACS2 (Fig. S4b).
MoIug4 contains an N-terminal secretion signal and a C-terminal zinc-finger domain predicted by the Simple Modular Architecture Research Tool (SMART) database (Fig. 2d). Given that the zinc finger domains are widely dispersed and actively participate in sequence-specific binding to DNA/RNA in eukaryotic genomes, as well as being involved in protein–protein recognition (Kim et al., 2020), we hypothesize that MoIug4 binds to the promoter of OsEIN2 or OsEIN3 to regulate the ET pathway.
In order to test this hypothesis, we performed a genome-wide ChIP-Seq assay (Table S2). Based on the data from overexpression (MoIUG4-OX) rice lines in TP309, we found that MoIug4 binds to the promoter region of OsEIN2 (Fig. 2e). We further predicted the putative cis-element of MoIug4 with OsEIN2 by Meme (http://meme-suite.org/tools/meme) (Figs 2f, S5). To verify the binding and prediction, we generated DNA fragments containing the OsEIN2 promoter sequence. As both OsEIN3 and OsETR1 were upregulated during the interaction between rice and Guy11 compared with the ΔMoiug4 mutant, we also generated DNA containing promoter sequences of OsEIN3 and OsETR1. With the NPR1 promoter as a control, only the DNA band of the OsEIN2 promoter was retarded by purified MoIug4, and this retardation increased in proportion to the amount of MoIug4 added (Figs 2g, S6). Moreover, we generated the putative cis-elements deletion promoter of OsEIN2 with digoxin (DIG)-labeled oligomers with the binding site, mutated its binding site and performed in vitro binding assays. We found that the band of oligomers with OsEIN2 promoter migrated slower than those of free DNA, and the mobility decreased with increasing concentration of MoIug4. When the putative cis-elements were deleted, the band of oligomer migrated as fast as free DNA (Fig. 2f,h). These results indicated that MoIug4 specifically targets the OsEIN2 promoter region.
MoIug4 interacts with rice OsAHL1
In order to better understand the regulation mechanism of MoIug4 in the ET signaling pathway, we performed Y2H screens using an M. oryzae-infected rice cDNA library (Fig. S7). Based on the cis-element of MoIug4, we focused on an AT-hook DNA-binding protein, OsAHL1 (LOC_Os 08g02490). By a co-IP assay in rice protoplasts, we found that OsAHL1 interacts with MoIug4 in vivo (Fig. 3a). As OsAHL1 contains two AT-hook DNA-binding domains in its N-terminal region (Fig. 3b), implying that it may have a role in transcriptional regulation. Truncated OsAHL1 mutants were obtained: OsAHL1NT containing two AT-hook DNA-binding domains and OsAHL1CT containing a Pfam:DUF296 domain. The Y2H assay showed that MoIug4 interacts with OsAHL1NT but not OsAHL1CT (Fig. 3c). We also performed an in vitro pull-down assay using recombinant proteins produced in E. coli that showed MoIug4-HIS enrichment in glutathione resins bound to OsAHL1-GST and OsAHL1NT-GST (Figs 3d, S8a).
Fig. 3.
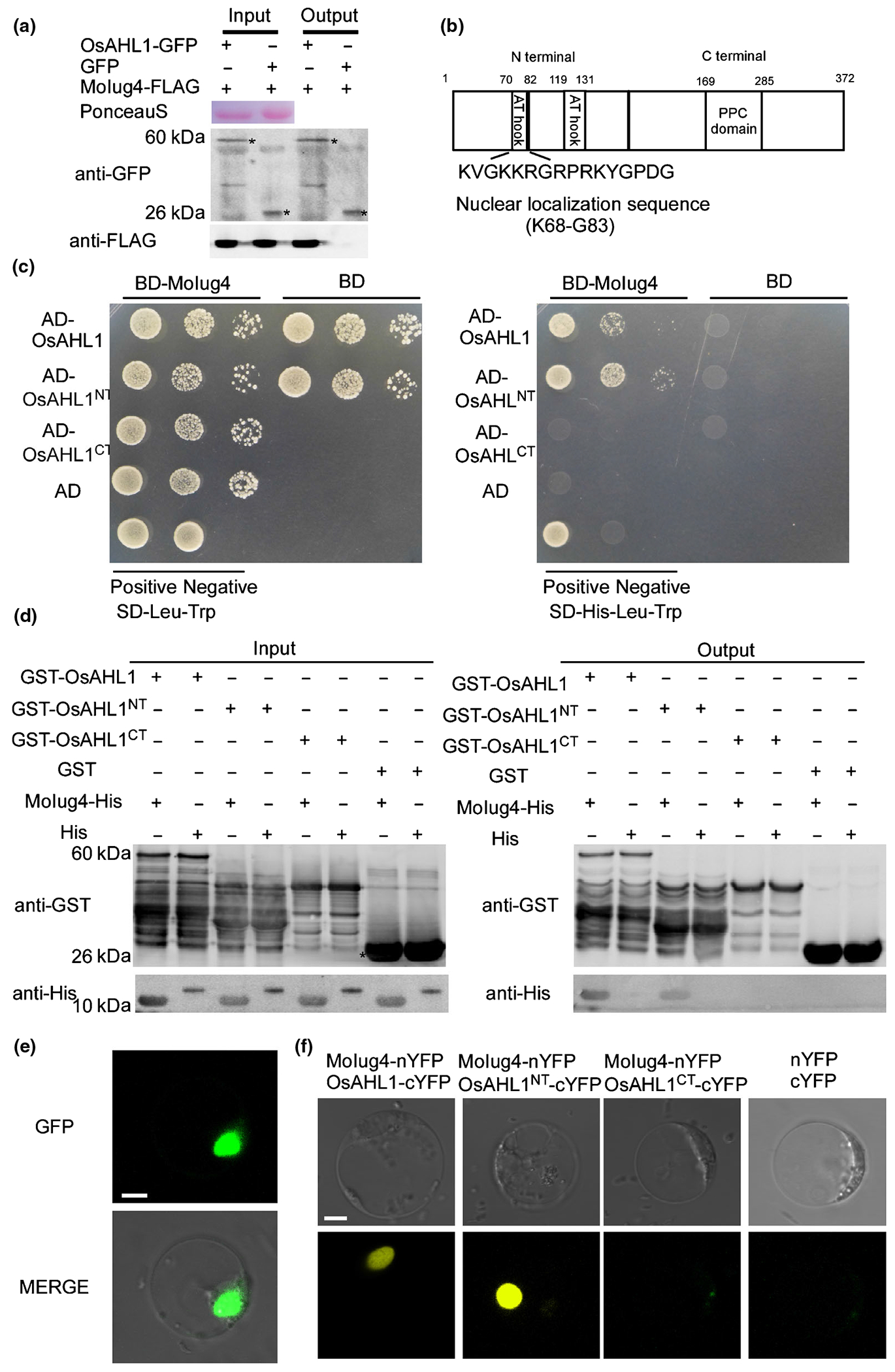
Identification of rice AT-hook proteins interacting with MoIug4. (a) Co-immunoprecipitation (Co-IP) assay. Both pBIN-OsAHL1-GFP and pXZ-MoIug4-FLAG were transformed into the protoplast of rice cells (GFP, green fluorescent protein). Western blot analysis of total proteins (T) extracted from the protoplasts: suspension proteins (S) and elution proteins (E) eluted from anti-GFP beads. The presence of MoIug4 and OsAHL1 was detected with anti-GFP and anti-FLAG antibodies, respectively. pBIN-GFP was used as the negative control. Asterisks represent the indicated proteins. (b) Characterization of different domains of OsAHL1. Nuclear localization sequence located at 68–83 aa position. The AT-hook domain is located at 70–82 and 119–131 aa position. (c) Yeast two-hybrid analysis of interactions between OsAHL1, OsAHL1NT (contained two AT-hook DNA-binding domain), and OsAHL11CT (contained Pfam:DUF296 domain) and MoIug4. cDNA was inserted into pGADT7 and pGBKT7, respectively. The pair of plasmids pGBKT7-Lam and pGADT7-T were used as negative controls. The pair of plasmids pGBKT7-(53+) and pGADT7-T were used as positive controls. Yeast cells grown on synthetic dextrose (SD) medium lacking His-Leu-Trp were investigated against positive and negative controls as indicated. Plates were incubated at 30°C for 3 d before being photographed. (d) Glutathione S-transferase (GST)-pull-down assay. In vitro GST-pull down assays confirm the interaction between MoIug4 and OsAHL1. The recombinant GST-OsAHL1, GST-OsAHL1NT, GST-OsAHL1′CT or GST bound to glutathione Sepharose beads were incubated with Escherichia coli cell lysate containing His-MoIug4 or His. Eluted proteins were analyzed by immunoblot (IB) with monoclonal anti-His and anti-GST antibodies. (e) Localization of OsAHL1 in rice cells. Bar, 5 μm. (f) Bimolecular fluorescence (BiFC) assays for the interaction between MoIug4 and OsAHL1. Rice protoplasts expressed MoIug4-nYFP and OsAHL1-cYFP, MoIug4-nYFP and OsAHL1NT-cYFP, and MoIug4-nYFP and OsAHL1CT-cYFP were observed under a confocal fluorescence microscope (Zeiss LSM710, ×63 oil); the nYFP-cYFP pair were used as negative controls. YFP, yellow fluorescent protein. Bar, 5 μm.
Protein interactions were further confirmed by the BiFC assay. The MoIUG4-NYFP and OsAHL1-CYFP, OsAHL1NT-CYFP and OsAHL1CT-CYFP fusion constructs were introduced into rice protoplasts, with the empty vectors serving as negative controls. The recombined YFP fluorescence signal was detected in the corresponding protein pairs’ nuclei (Figs 3e, S8b,c).
Overexpression of OsAHL1 in rice enhances resistance to M. oryzae
In order to characterize OsAHL1 in rice, we infected the susceptible ecotype TP309 with Guy11. Real-time PCR assays showed that OsAHL1 was upregulated during the early stages of infection, with a maximal expression level at 24 hpi (Fig. 4a). We first generated the transgenic OsAHL1 CRISPR gene deletion (OsAHL1-KO) and overexpression (OsAHL1-OX) rice lines in the TP309 background (Fig. S9a). To further determine if OsAHL1 plays a role in resistance towards M. oryzae, OsAHL1-KO, OsAHL1-OX and TP309 rice seedlings were (respectively) sprayed with WT Guy11 conidia. The production of fewer lesions on OsAHL1-OX rice lines at 7 dpi (Fig. 4b,d) indicated that OsAHL1 positively regulates rice resistance to M. oryzae. In infection, OsAHL1-OX rice lines showed strong resistance to blast, with punctate and significantly reduced lesion areas, whereas OsAHL1-KO and MoIUG4-OX lines were more susceptible (Fig. 4c,e). We also examined the role of MoIUG4 in pathogenicity by overexpressing (MoIUG4-OX lines) in the TP309 background. Consistent with lesion areas, M. oryzae infection was more abundant on MoIug4-OX lines than on the TP309 line (Figs 4b,c, Sl0), suggesting that MoIUG4-OX enhances the susceptibility of rice to M. oryzae infection. We also examined penetration and invasive hyphal extension in rice sheath cells. After incubation with conidia suspension for 36 h, > 76% of Guy11 IH spread freely into adjacent cells in OsAHL1-KO and MoIUG4-OX lines. By contrast, IH were restricted in primary infected cells when OsAHL1-OX was infected with Guy11 (Fig. 4f), suggesting that OsAHL1 plays a critical role during rice interaction with M. oryzae.
Fig. 4.
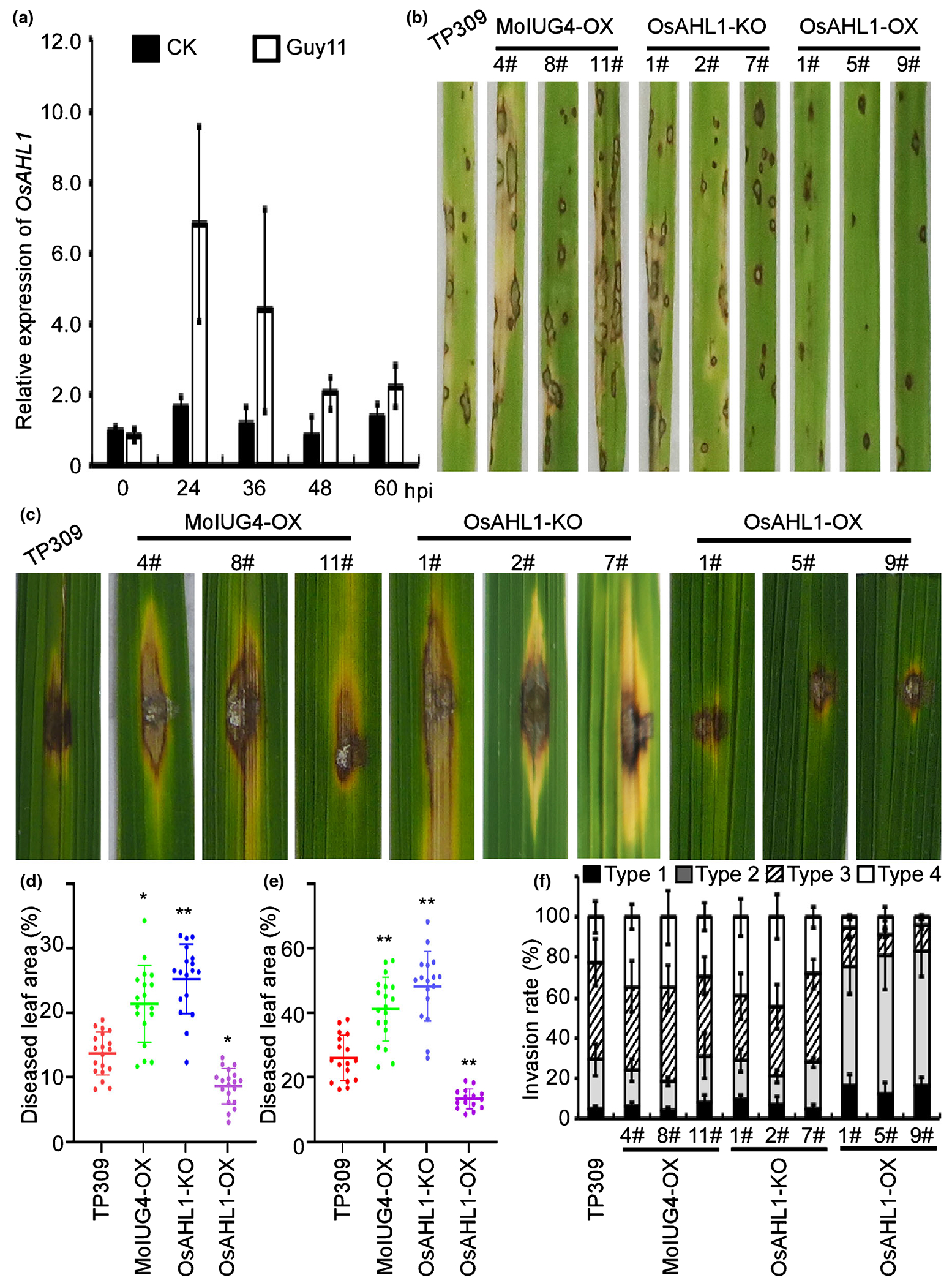
Oryza sativa OsAHL1 positively regulates rice resistance to Magnaporthe oryzae. (a) Expression of OsAHL1 at different stages of M. oryzae infection was determined by quantitative real-time (qRT)-PCR. Two-week-old leaves from cv. CO-39 were inoculated with M. oryzae. CK represents H2O control. Error bars represent SD. (b) Blast resistance of OsAHL1 CRISPR knockout mutant (OsAHL1KO), MoIUG4 overexpression mutant (MoIug4-OX) and OsAHL1 overexpression mutant (OsAHL1-OX) plants using spraying inoculation. (c) Leaves of 4-wk-old plants were inoculated using the punch method. Photos were taken at 6 d post-inoculation (dpi). (d) The diseased leaf area infected with Guy11 through spraying inoculation was assessed by ImageJ. Lesions were photographed and measured or scored at 7 dpi. Error bars represent SD. Student’s t-test: *, P < 0.05; **, P < 0.01. (e) Diseased leaf areas by punch injection were assessed by ImageJ. Lesions were photographed and measured or scored at 6 dpi. Error bars represent SD. Student’s t-test: **, P < 0.01. (f) Excised rice sheaths from 3-wk-old rice seedlings were inoculated with conidial suspension (1 × 105 spores ml−1). Detailed observation and statistics for infectious growth in rice sheath cells at 36 h post-inoculation (hpi). Appressorium penetration sites (n = 100) were observed and invasive hyphae (IH) were rated from type 1 to 4. The experiment was repeated three times. Error bars represent SD.
MoIug4 exhibits a higher affinity to the OsEIN2 promoter than that of OsAHL1
Because OsAHL1 contains two AT-hook DNA binding domains, we hypothesized that OsAHL1 might function as a transcription factor. To test this, we first generated the OsAHL1, OsAHL1NT and OsAHL1CT constructs using the pGBKT7 vector. The Y2H assay showed that yeast containing OsAHL1 and OsAHL1CT were grown on synthetic dextrose (SD) medium lacking leucine (Leu), tryptophan (Trp) and histidine (His) (Fig. 5a). The EMSA assay further showed that the AT-hook DNA binding domain of OsAHL1 binds with the promoter of OsEIN2 (Figs 5b, S11). We further mutated the MoIug4-binding element on the promoter of OsEIN2, and found that DNA containing the promoter sequence of OsEIN2 was retarded by the addition of the purified OsAHL1 protein and this retardation increased drastically as the amount of OsAHL1 increased. By contrast, the mutated promoter of OsEIN2 showed a low affinity with OsAHL1 (Fig. 5c). Given that both MoIug4 and OsAHL1 bind to the same cis-element of OsEIN2, we conducted in vitro pull-down assay using OsAHL1-GST and MoIug4-HIS proteins. With the decreasing concentration of MoIug4 or OsAHL1 added to the reaction system, the enrichment of the OsEIN2 promoter was detected by qRT-PCR. The input OsEIN2 promoter (pro-OsEIN2) was used as a control. After elution from anti-GST or anti-HIS beads, both OsAHL1-bound and MoIug4-bound resin was gradually decreased. However, the rate and amount of pro-OsEIN2 reduction of OsAHL1-bound were significantly faster than MoIug4-bound (Fig. 5d), suggesting that MoIug4 has a higher affinity to the OsEIN2 promoter than that of OsAHL1.
Fig. 5.
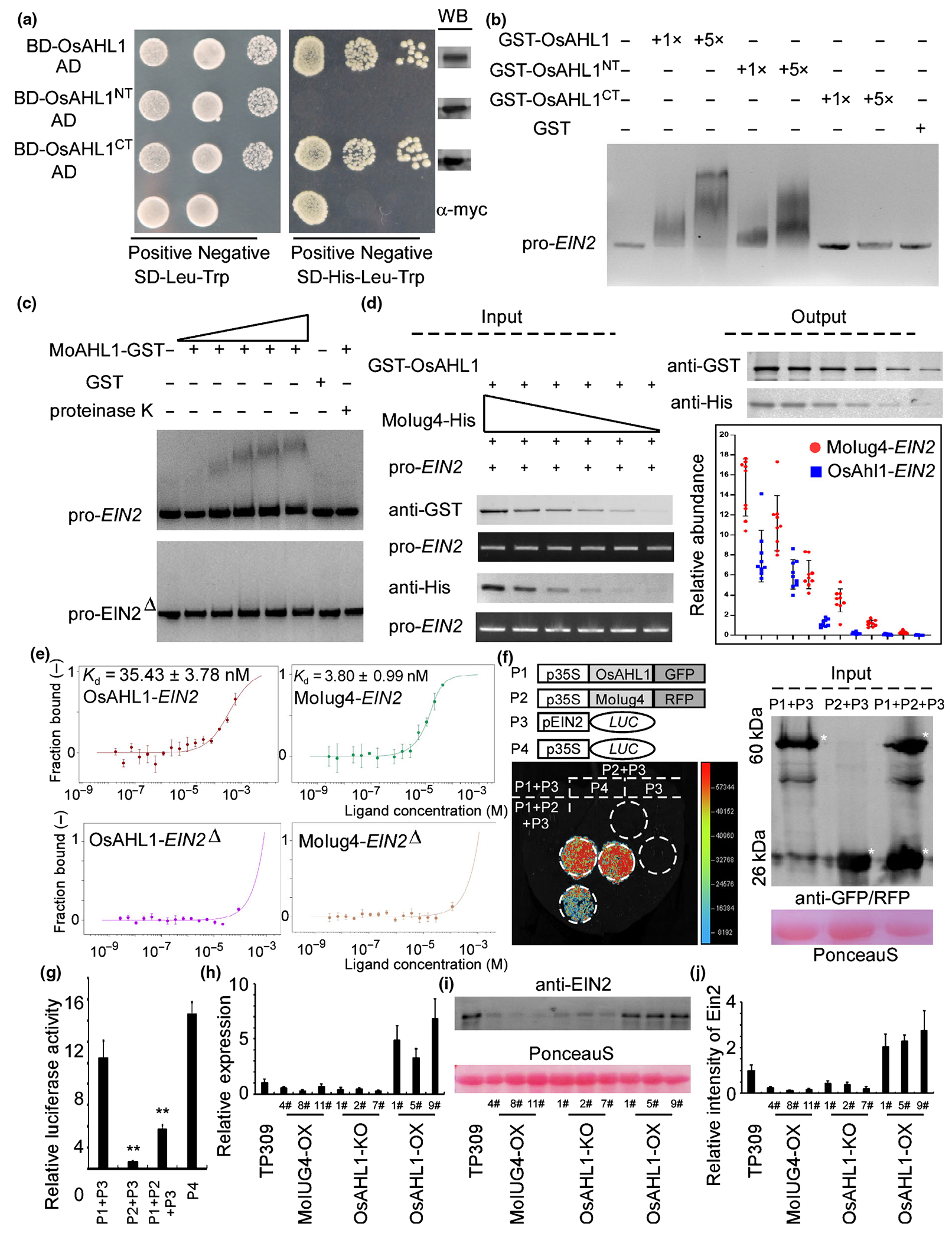
Normal Oryza sativa OsAHL1 transcription is required for immunity that also is controlled by Magnaporthe oryzae MoIug4. (a) Activity of OsAHL1 in yeast. The OsAHL1, OsAHL1NT and OsAHL1CT fragments were fused with the DNA binding domain (BD), respectively, and the derived plasmids were transformed into yeast. Protein expression in yeast cells was examined by Western blot (WB) using anti-Myc. (b) EMSA assay: full-length OsEIN2 promoter sequence was incubated with purified GST-OsAHL1, GST-OsAHL1NT, GST-OsAHL1CT and GST proteins (rightmost lanes) (GST, glutathione S-transferase). (c) Full-length promoter and also the cis-element mutation promoter (EIN2Δ, which includea the element TGTACACAG in the promoter of OsEIN2) of OsEIN2 were incubated without (leftmost lane) or with (second to the sixth lane with increasing amounts of OsAHL1) purified OsAHL1 and GST proteins (seventh lane). The proteinase K was added after the incubation of OsAHL1 with DNA (rightmost lane). DNA–protein complexes were separated by electrophoresis on a 1.2% agarose gel and stained by ethidium bromide. (d) OsEIN2 promoter exhibits a higher affinity with MoIug4 than OsAHL1 in the in vitro assay. Co-precipitation of the OsEIN2 promoter with total MoIug4 or OsAHL1 protein extracts from Escherichia coli in different gradient dilutions (input) was examined by WB before and after affinity purification with anti-GST or anti-His antibodies. The MoIug4- and OsAHL1-binding of the OsEIN2 promoter (output) was detected by quantitative real-time (qRT)-PCR with the input OsEIN2 promoter as a control. Error bars indicate SD of biological replicates. (e) Microscale thermophoresis (MST) assay shows that MoIug4 forms a complex with the OsEIN2 promoter with Kd, 3.80 nM (green). OsAHL1 shows a lower affinity to the OsEIN2 promoter (Kd, 35.43 nM, red). Deletion of the cis-element leads to the disassociation in the OsAHL1–OsEIN2 pair (purple) and MoIug4–OsEIN2 pair (orange). Error bars represent SD. (f) Split-luciferase assays were performed to test the interaction of MoIug4 and OsAHL1 with the OsEIN2 promoter in vivo. OsEIN2 promoter was fused with LUC. 35S-LUC was used as a positive control (+). Nicotiana benthamiana leaves were used to measure the LUC activity 48 h after co-expression of indicated proteins using the chemiluminescent Imaging system. Anti-GFP/RFP was used to evaluate the expression of OsAHL1 and MoIug4 in N. benthamiana leaves (GFP and RFP, green and red fluorescent protein). Asterisk represents the indicated proteins. (g) Rice protoplasts were used to measure the luciferase activity 24 h after co-expression of indicated proteins using the GLOMAX96 microplate luminometer. Student’s t-test: *, P < 0.01. (h) Expression of OsEIN2 was detected by qRT-PCR in MoIUG4-OX, OsAHL1-KO and OsAHL1-OX lines. TP309 was used as a negative control. Error bars represent SD. (i, j) Total proteins were extracted from MoIUG4-OX, OsAHL1-KO and OsAHL1-OX lines. TP309 was used as a negative control. OsEIN2 concentrations were evaluated by anti-EIN2. Error bars represent SD.
OsAHL1–OsEIN2 and MoIug4–OsEIN2 interactions were further confirmed by the microscale thermophoresis (MST) experiment, in which the OsEIN2 promoter was found to bind to MoIug4-HIS with high affinity (Kd, 3.80 nM) (Fig. 5e). In addition, the bound assay exhibited a 10-fold lower affinity (Kd, 35.43 nM) of OsEIN2 to OsAHL1-GST (Fig. 5e), suggesting that MoIug4 binds with the OsEIN2 promoter with a higher affinity. Moreover, when the cis-element on the promoter of OsEIN2 was deleted, both MoIug4 and OsAHL1 showed an extremely low affinity with the mutational OsEIN2 (Fig. 5e).
Finally, a luciferase reporter analysis was carried out to examine whether MoIug4 and OsAHL1 regulate the expression of OsEIN2 in a competitive manner. When co-incubated MoIug4 and OsAHL1 in N. benthamiana leaves and rice protoplast, we found that the transcriptional activity of OsAHL1 was drastically decreased in comparison with OsAHL1, indicating that MoIug4 may acts as a competitor in OsAHL1 binding to the OsEIN2 promoter. The results showed that OsAHL1, but not MoIug4, directly regulates the transcription of OsEIN2 (Fig. 5f,g). We examined the expression of OsEIN2 in these transgenic lines. qRT-PCR assays showed that the expression of OsEIN2 was significantly decreased in the MoIUG4-OX lines (Fig. 5h). We further evaluated the accumulation of OsEIN2 by immunoblotting using the OsEIN2-specific antibody (AS12-1865; Agrisera, Vannas, Sweden) with the TP309 background as a control. Our data showed that OsAHL1-OX rice lines exhibit enhanced OsEIN2. By contrast, the MoIUG4-OX lines showed a decreased accumulation of OsEIN2, consistent with the transcription study results (Fig. 5i,j).
As OsEIN2 was reported to regulate OsRBOHA/B expression (Yang et al., 2017), we detected the expression of OsRBOHA/B in MoIUG4-OX, OsAHL1-KO and OsAHL1-OX rice, and found that the expression of OsRBOHA/B was significantly inhibited in MoIUG4-OX rice (Fig. S12a). To further understand the function of MoIug4 on regulating the ET signaling pathway, we evaluated the generation of ET in MoIUG4-OX and OsAHL1-OX rice. The results showed that although ET also can be produced in the MoIUG4-OX and OsAHL1-KO lines, ET production is significantly suppressed compared to that in OsAHL1-OX (Fig. S12b). Because ET production is inhibited when M. oryzae interacts with MoIUG4-OX rice, we used 2-Chloroethylphosphonic acid (etephone) to treat MoIUG4-OX rice and induce the production of ET. After evaluating the expression of OsRBOHA/B, the results exhibited that the expression of these genes regulated by OsEIN2 was suppressed in MoIUG4-OX lines when compared with TP309 lines (Fig. S12c), suggesting that MoIug4 inhibits the function of OsEIN2 in regulating the ET signaling pathway. Taken together, we concluded that the overexpression of MoIUG4 leads to a competition binding to the OsEIN2 promoter that inhibits OsAHL1-mediated transcription resulting in subversion in immunity.
Discussion
Plants have developed a complex immune system to defend against invasion by microbes, which relies on elaborate signaling networks regulated by phytohormones. In turn, microbes have evolved complex strategies to manipulate plant defense or control plant cell function for their benefit. For example, pathogenic microbes deliver a variety of effectors that target different components of host signaling pathways to manipulate plant defense and promote colonization (Patkar et al., 2015; Funato et al., 2020). Ethylene (ET) plays a key role in the host responding to biotic and abiotic stresses. Ethylene is sensed by a family of endoplasmic reticulum (ER)-localized receptors, which transduce signaling to a Raf-like Ser/Thr protein kinase OsCTR1. OsCTR1 further acts upstream of the membrane protein OsEIN2, a central transducer of ET signaling. OsEIN2, OsEIN3 and OsEIL1 function as master transcription factors to activate the expression of targets such as ERF1 that regulate many ET response genes (Liu et al., 2020). In rice, several components were characterized that show positive regulation in the resistance to M. oryzae infection. Helliwell et al. (2016) introduced the ACC synthase gene OsACS2 into the rice and found that transgenic rice lines exhibit strong disease resistance to M. oryzae, linking ET biosynthesis to disease resistance. Moreover, using OsEIN2 and OsEIL1 transgenic plants, previous studies demonstrated that the activation of ET signaling also enhances the resistance of rice to M. oryzae (Yang et al., 2017; Liu et al., 2021).
In order to inhibit ethylene-derived host immunity, pathogens secrete various effectors. In Phytophthora sojae, PsAvh238 promotes infection by destabilizing type 2 GmACSs and reducing ET production that attenuates plant defense responses (Yang et al., 2019a). A Xanthomonas type III effector XopD deSUMOylates an ET-responsive transcription factor to suppress ET accumulation in Arabidopsis thaliana (Kim et al., 2013). Recent work showed that SsERP1 inhibits plant ET signaling to promote the infection of Sclerotinia sclerotiorum (Fan et al., 2021). In Ustilago maydis, a novel effector Jsi1 interacts with Topless corepressor that hijacks ethylene signaling in plants (Darino & Chia, 2021). As a secreted effector of Glomus intraradices, SP7 targets the Medicago truncatula ET-responsive transcription factor ERF19 to suppress plant defense. Finally, the expression of the MoSP7 protein in M. oryzae increases the biotrophic phase in rice roots (Kloppholz et al., 2011).
During the interaction between M. oryzae and rice, effectors that function on either ET biosynthesis or the ET signaling pathway remain unknown. Our results shed new insight into the virulence strategy of M. oryzae by suppressing the ET signaling pathway. In our study, MoIug4 specifically suppresses the ET-mediated signaling pathway of rice to benefit M. oryzae infection. The three phytohormones SA, JA and ET are known to play major roles in regulating plant defense responses against various pathogens, pests and abiotic stresses (Loake & Grant, 2007; Liu et al., 2020). JA and ET usually are associated with defenses against necrotrophic pathogens and herbivorous insects (Kong et al., 2017). We showed that the disruption of MoIUG4 causes the accumulation of ROS around the infection site that is considered as the early defense response. Ethylene also can function positively in rice immunity, drawing on evidence from ET-overproducing rice transformants that show increased resistance to M. oryzae (Zhu et al., 2016). Previous studies also showed that OsEin2 modulates disease resistance to M. oryzae by positively regulating the expression of the OsRHOB genes, which subsequently leads to the accumulation of ROSs (Yang et al., 2017). In contrast to ET signaling, SA generally is involved in the activation of defense responses against biotrophic and hemibiotrophic pathogens and the establishment of systemic acquired resistance (Zhang et al., 2019).
Mutants with a defect in the accumulation of SA or that are insensitive to SA show enhanced susceptibility to biotrophic and hemibiotrophic pathogens (Park et al., 2007). However, M. oryzae does not induce SA production in rice to any significant degree (Wang & Wang, 2018; Yuan et al., 2021). Silencing of the SA-responsive marker gene OsNPR1 results in the suppression of pathogenesis-related (PR) genes and increased infection of M. oryzae in rice (Feng et al., 2011). Overexpression of OsNPR1 triggers the expression of PR genes in transgenic rice plants and confers resistance to M. oryzae (Feng et al., 2011). These results indicated that spatiotemporal functions of plant hormones that inhibit host-derived immunity are not absolute. As MoIug4 targets various genes’ promoters according to the chromatin immunoprecipitation (ChIP)-seq data, we do not rule out the possibility that MoIug4 may be interacting with other rice targets in regulating pathogen-associated molecular pattern (PAMP)-triggered immunity (PTI).
As a cytoplasm effector, MoIug4 accumulates in the biotrophic interfacial complex (BIC) during the early stage of the infection and transfers it to the host cell, where it regulates host-derived immunity. We provided evidence that MoIug4 disruption significantly induces the expression of ET signaling genes affecting transcriptional reprogramming in the host. Transcriptional reprogramming is a critical strategy for host plants to initiate defense against pathogens and is likewise a significant target for pathogen effectors. Histone acetylation is an essential epigenetic marker linked to transcription regulation, and GCN5-mediated histone acetylation was shown to play an essential role in plant development and responses to environmental stresses (Ge et al., 2007; Dong et al., 2015). A P. sojae effector, PsAvh23, binds to ADA2 and disrupts the formation of the ADA2–GCN5 complex that suppresses host ADA2/GCN5-mediated H3K9ac to enhance susceptibility (Kong et al., 2017). Except for the regulation of histone acetylation, another P. sojae effector, PsCRN108, targets the heat shock protein (HSP) promoters to inhibit the association of the heat shock element (HSE) with the plant heat shock transcription factor AtHsfA1a, which initializes HSP gene expression in response to stress (Kotchoni & Gachomo, 2006). In M. oryzae, AvrPiz-t binds to APIP5 and suppresses its transcriptional activity and protein accumulation, which causes effector-triggered necrosis (Wang et al., 2016). However, the mechanism for this remains unknown. Recently, Kim et al. (2020) showed that several nuclear effectors reprogram the transcription of host-defense genes by directly targeting promoters. We revealed that MoIug4 does not belong to any MoHTR genes even though its function overlaps with MoHTR1/2-regulating genes, such as Os03g0161800, Os04g0556300, Os07g0596300 and Os07g0154100.
We have identified that MoIug4 hijacks the host-defense gene promoter in a more complex manner. MoIug4 is an effector targeting the host (rice and barley) ET signaling pathway and it binds to the promoter of OsEIN2 and HvEIN2 (Figs 2g, S13), acting as a transcription repressor. MoIug4 also interacts with OsAHL1. The self-transactivation activity and luciferase reporter analyses indicated that OsAHL1 displays a robust transcriptional activity. Consistent with the premises that MoIug4, OsAHL1 and the OsEIN2 promoter interact with each other, and that MoIug4 shows no effects on the protein stability of OsAHL1 (Fig. S14), we assessed the affinity level of the MoIug4–OsEIN2 and OsAHL1–OsEIN2 pairs in vitro (Fig. 5d,e), and showed that MoIug4 inhibits the function of OsAHL1 (Fig. 5f,g). We further performed a yeast-two-hybrid (Y2H) assay by transforming free MoIug4 (lack of active domain) and BD-OsAHL1 in yeast and found that yeast still grows (Fig. S15). This result indicated that OsAHL1 may function on the transcription of various target genes independent of MoIug4, or that Molug4 inhibition may depend on specific plant co-repressors. As MoIug4 functions as a host immunity repressor located in the nucleus (Figs 1h, 2c) but lacked a definite nuclear localization signal which was predicted by NucPred (https://nucpred.bioinfo.se/cgi-bin/single.cgi), we hypothesized that the host may assist MoIug4 in translocation to the nucleus, although this remains to be proven. To effectively inhibit the defects caused by MoIug4 in the nucleus, the host may achieve to reunite OsAHL1 on these promoters by inducing the nuclear export or inhibiting the nuclear localization of MoIug4. However, the deletion of OsAHL1 did not change the nuclear localization of MoIug4 in planta (Fig. S16a). Given that MoIug4 binds to the promoter of OsEIN2 with a higher affinity than OsAHL1 that represses OsEIN2 expression (Figs 5e–g, S16b), it is plausible that OsAHL1 also is a client of MoIug4 in the nucleus leading to the better positioning of the effector in the promoter region of the target gene.
Based on all of the evidence obtained, we conclude that MoIug4 binds with the promoter of OsEIN2 with a higher affinity than OsAHL1 and functions as a transcription factor to repress OsEIN2 expression in planta. Our findings expand the current understanding of how microbes use specific weapons to target various components of host cells to subvert host immunity and promote infection (Fig. 6).
Fig. 6.
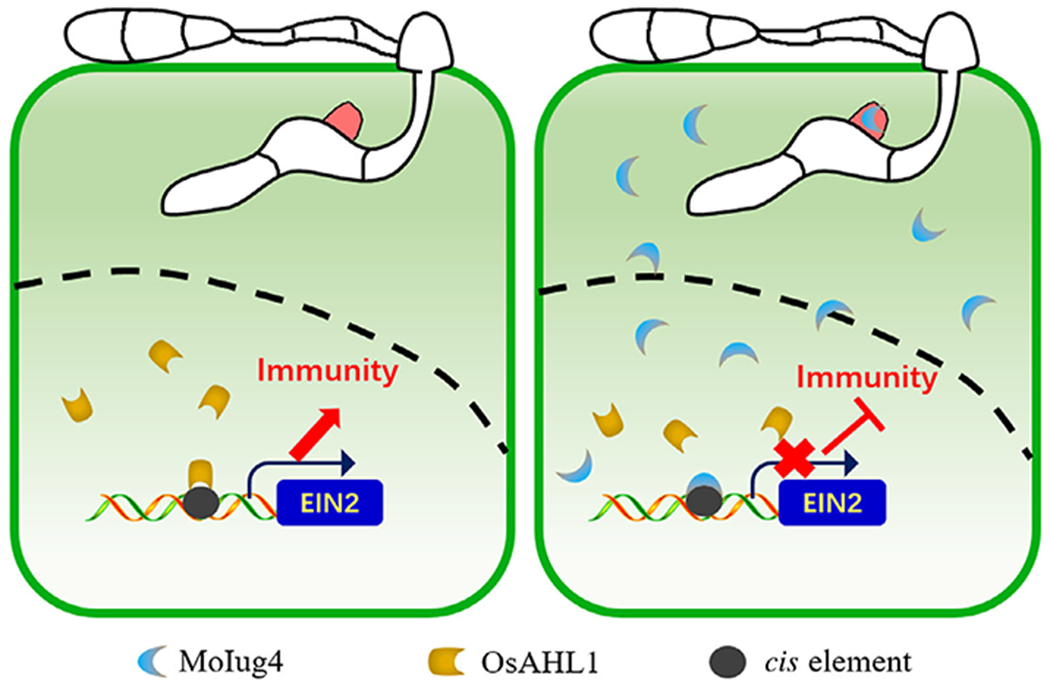
Schematic summary of the role of MoIug4 and OsAHL1 during the interaction between Magnaporthe oryzae and rice (Oryza sativa). Cytoplasmic effector MoIug4 secreted from biotrophic interfacial complex (BIC) during infection is translocated into rice cells. MoIug4 interacts with an AT-hook protein, OsAHL1, which functions on the positive regulation of the ethylene (ET) signaling pathway component OsEIN2 in transcription that enhances resistance to fungi. Once MoIug4 is transferred into rice cells, MoIug4 binds to the promoter of OsEIN2 with an affinity higher than that of OsAHL1 to suppress the expression of OsEIN2 leading to inhibition of OsEIN2-mediated immunity. Black dashed line, nuclear membrane.
Supplementary Material
Fig. S1 Southern blot analysis of the Moiug4 mutant.
Fig. S2 Localization of MoIug4 fused with NLS during infection in rice cells.
Fig. S3 Localization of MoIug4 expressed in Nicotiana benthamiana.
Fig. S4 MoIug4 is important for the suppression of ethylene production in rice during infection by Magnaporthe oryzae.
Fig. S5 Enriched sequences from the Chip-Seq analysis were exhibited.
Fig. S6 MoIgu4 specifically binds with the OsEIN2 promoter.
Fig. S7 Putative MoIug4-interacting proteins identified by screening the rice cDNA library.
Fig. S8 Controls for the pull-down and BIFC assays.
Fig. S9 Validation of transgenic lines.
Fig. S10 OsAHL1 is important for the rice resistance to Magnaporthe oryzae.
Fig. S11 The OsAHL1 N-terminal domain binds with the OsEIN2 promoter.
Fig. S12 MoIug4 and OsAHL1 are important for ethylene production and ethylene signaling transduction during interaction between rice and Magnaporthe oryzae.
Fig. S13 MoIug4 showed no effects on the protein stability of OsAHL1.
Fig. S14 MoIug4 showed no effects on the protein stability of OsAHL1.
Fig. S15 Activity of OsAHL1 in yeast.
Fig. S16 Localization and function of MoIug4 in OsAHL1-KO lines.
Table S1 Identification of MoIug4 in Guy11.
Table S2 Information of ChIP-seq analysis of MoIug4.
Acknowledgements
This research was supported by the program of NSFC-DFG (grant no. 31861133017), Youth Program for Natural Science Foundation of Jiangsu Province (BK20190512) and NSFC (grant no. 31972979). Research in the Ping Wang lab was supported by the National Institutes of Health (US) award nos. AI156254 and AI168867.
Footnotes
Supporting Information
Additional Supporting Information may be found online in the Supporting Information section at the end of the article.
Data availability
The data that support the findings of this study are available in the Supporting Information of this article.
References
- Ausubel FM. 2005. Are innate immune signaling pathways in plants and animals conserved? Nature Immunology 6: 973–979. [DOI] [PubMed] [Google Scholar]
- Bari R, Jones JD. 2009. Role of plant hormones in plant defence responses. Plant Molecular Biology 69: 473–488. [DOI] [PubMed] [Google Scholar]
- Boller T, He SY. 2009. Innate immunity in plants: an arms race between pattern recognition receptors in plants and effectors in microbial pathogens. Science 324: 742–744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolwell GP, Davies DR, Gerrish C, Auh CK, Murphy TM. 1998. Comparative biochemistry of the oxidative burst produced by rose and French bean cells reveals two distinct mechanisms. Plant Physiology 116: 1379–1385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chanclud E, Kisiala A, Emery NR, Chalvon V, Ducasse A, Romiti-Michel C, Gravot A, Kroj T, Morel JB. 2016. Cytokinin production by the rice blast fungus is a pivotal requirement for full virulence. PLoS Pathogens 12: e1005457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen XL, Shi T, Yang J, Shi W, Gao X, Chen D, Xu X, Xu JR, Talbot NJ, Peng YL. 2014. N-glycosylation of effector proteins by an alpha-1,3-mannosyltransferase is required for the rice blast fungus to evade host innate immunity. Plant Cell 26: 1360–1376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chi MH, Park SY, Kim S, Lee YH. 2009. A novel pathogenicity gene is required in the rice blast fungus to suppress the basal defenses of the host. PLoS Pathogens 5: e1000401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darino M, Chia KS. 2021. Ustilago maydis effector Jsi1 interacts with Topless corepressor, hijacking plant jasmonate/ethylene signaling. New Phytologist 229: 3393–3407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong Y, Li Y, Zhao M, Jing M, Liu X, Liu M, Guo X, Zhang X, Chen Y, Liu Y et al. 2015. Global genome and transcriptome analyses of Magnaporthe oryzae epidemic isolate 98-06 uncover novel effectors and pathogenicity-related genes, revealing gene gain and lose dynamics in genome evolution. PLoS Pathogens 11: e1004801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan H, Yang W, Nie J, Zhang W, Wu J, Wu D, Wang Y. 2021. A novel effector protein Sserp1 inhibits plant ethylene signaling to promote Sclerotinia sclerotiorum infection. Journal of Fungi 7: 825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng JX, Cao L, Li J, Duan CJ, Luo XM, Le N, Wei H, Liang S, Chu C, Pan Q. 2011. Involvement of OsNPR1/NH1 in rice basal resistance to blast fungus Magnaporthe oryzae. European Journal of Plant Pathology 131: 221–235. [Google Scholar]
- Funato K, Riezman H, Muniz M. 2020. Vesicular and non-vesicular lipid export from the ER to the secretory pathway. Biochimica et Biophysica Acta: Molecular and Cell Biology of Lipids 1865: 158453. [DOI] [PubMed] [Google Scholar]
- Ge X, Li GJ, Wang SB, Zhu H, Zhu T, Wang X, Xia Y. 2007. AtNUDT7, a negative regulator of basal immunity in Arabidopsis, modulates two distinct defense response pathways and is involved in maintaining redox homeostasis. Plant Physiology 145: 204–215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giraldo MC, Dagdas YF, Gupta YK, Mentlak TA, Yi M, Martinez-Rocha AL, Saitoh H, Terauchi R, Talbot NJ, Valent B. 2013. Two distinct secretion systems facilitate tissue invasion by the rice blast fungus Magnaporthe oryzae. Nature Communications 4: 1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giraldo MC, Valent B. 2013. Filamentous plant pathogen effectors in action. Nature Reviews Microbiology 11: 800–814. [DOI] [PubMed] [Google Scholar]
- Grant M, Brown I, Adams S, Knight M, Ainslie A, Mansfield J. 2000. The RPM1 plant disease resistance gene facilitates a rapid and sustained increase in cytosolic calcium that is necessary for the oxidative burst and hypersensitive cell death. The Plant Journal 23: 441–450. [DOI] [PubMed] [Google Scholar]
- Guo M, Chen Y, Du Y, Dong Y, Guo W, Zhai SU, Zhang H, Dong S, Zhang Z, Wang Y et al. 2011. The bZIP transcription factor MoAP1 mediates the oxidative stress response and is critical for pathogenicity of the rice blast fungus Magnaporthe oryzae. PLoS Pathogens 7: e1001302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helliwell EE, Wang Q, Yang Y. 2016. Ethylene biosynthesis and signaling is required for rice immune response and basal resistance against Magnaporthe oryzae infection. Molecular Plant–Microbe Interactions 29: 831–843. [DOI] [PubMed] [Google Scholar]
- Jones JD, Dangl JL. 2006. The plant immune system. Nature 444: 323–329. [DOI] [PubMed] [Google Scholar]
- Kim JG, Stork W, Mudgett MB. 2013. Xanthomonas type III effector XopD desumoylates tomato transcription factor SlERF4 to suppress ethylene responses and promote pathogen growth. Cell Host & Microbe 13: 143–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim JG, Stork W, Mudgett MB. 2016. Quantification of ethylene production in tomato leaves infected by Xanthomonas euvesicatoria. Bio-Protocol 6: e1723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim S, Kim C-Y, Park S-Y, Kim K-T, Jeon J, Chung H, Choi G, Kwon S, Choi J, Jeon J et al. 2020. Two nuclear effectors of the rice blast fungus modulate host immunity via transcriptional reprogramming. Nature Communications 11: 5845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kloppholz S, Kuhn H, Requena N. 2011. A secreted fungal effector of Glomus intraradices promotes symbiotic biotrophy. Current Biology 21: 1204–1209. [DOI] [PubMed] [Google Scholar]
- Kong L, Qiu X, Kang J, Wang Y, Chen H, Huang J, Qiu M, Zhao Y, Kong G, Ma Z et al. 2017. A Phytophthora effector manipulates host histone acetylation and reprograms defense gene expression to promote infection. Current Biology 27: 981–991. [DOI] [PubMed] [Google Scholar]
- Kotchoni SO, Gachomo EW. 2006. The reactive oxygen species network pathways: an essential prerequisite for perception of pathogen attack and the acquired disease resistance in plants. Journal of Biosciences 31: 389–404. [DOI] [PubMed] [Google Scholar]
- Li X, Gao C, Li L, Liu M, Yin Z, Zhang H, Zheng X, Wang P, Zhang Z. 2017. MoEnd3 regulates appressorium formation and virulence through mediating endocytosis in rice blast fungus Magnaporthe oryzae. PLoS Pathogens 13: e1006449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Liu X, Liu M, Wang Y, Zou Y, You Y, Yang L, Hu J, Zhang H, Zheng X et al. 2020. Magnaporthe oryzae auxiliary activity protein Moaa91 functions as chitin-binding protein to induce appressorium formation on artificial inductive surfaces and suppress plant immunity. mBio 11: e03304–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu M, Hu J, Zhang A, Dai Y, Chen W, He Y, Zhang H. 2021. Auxilin-like protein MoSwa2 promotes effector secretion and virulence as a clathrin uncoating factor in the rice blast fungus Magnaporthe oryzae. New Phytologist 230: 720–736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu M, Zhang S, Hu J, Sun W, Padilla J, He Y, Li Y, Yin Z, Liu X, Wang W et al. 2019. Phosphorylation-guarded light-harvesting complex II contributes to broad-spectrum blast resistance in rice. Proceedings of the National Academy of Sciences, USA 116: 17572–17577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X, Yang J, Qian B, Cai Y, Zou X, Zhang H, Zheng X, Wang P, Zhang Z. 2018. MoYvh1 subverts rice defense through functions of ribosomal protein MoMrt4 in Magnaporthe oryzae. PLoS Pathogens 14: e1007016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X, Zhang Z. 2021. A double-edged sword: reactive oxygen species (ROS) during the rice blast fungus and host interaction. The FEBS Journal. doi: 10.1111/febs.16171. [DOI] [PubMed] [Google Scholar]
- Liu X, Zhou Q, Guo Z, Liu P, Shen L, Chai N, Qian B, Cai Y, Wang W, Yin Z et al. 2020. A self-balancing circuit centered on MoOsm1 kinase governs adaptive responses to host-derived ROS in Magnaporthe oryzae. eLife 9: e61605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loake G, Grant M. 2007. Salicylic acid in plant defence–the players and protagonists. Current Opinion in Plant Biology 10: 466–472. [DOI] [PubMed] [Google Scholar]
- Mentlak TA, Kombrink A, Shinya T, Ryder LS, Otomo I, Saitoh H, Terauchi R, Nishizawa Y, Shibuya N, Thomma BP et al. 2012. Effector-mediated suppression of chitin-triggered immunity by Magnaporthe oryzae is necessary for rice blast disease. Plant Cell 24: 322–335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mittler R, Vanderauwera S, Suzuki N, Miller G, Tognetti VB, Vandepoele K, Gollery M, Shulaev V, Van Breusegem F. 2011. ROS signaling: the new wave? Trends in Plant Science 16: 300–309. [DOI] [PubMed] [Google Scholar]
- Park CH, Chen S, Shirsekar G, Zhou B, Khang CH, Songkumarn P, Afzal AJ, Ning Y, Wang R, Bellizzi M et al. 2012. The Magnaporthe oryzae effector AvrPiz-t targets the RING E3 ubiquitin ligase APIP6 to suppress pathogen-associated molecular pattern-triggered immunity in rice. Plant Cell 24: 4748–4762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park CH, Shirsekar G, Bellizzi M, Chen S, Songkumarn P, Xie X, Shi X, Ning Y, Zhou BO, Suttiviriya P et al. 2016. The E3 ligase APIP10 connects the effector AvrPiz-t to the NLR receptor Piz-t in rice. PLoS Pathogens 12: e1005529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park SW, Kaimoyo E, Kumar D, Mosher S, Klessig DF. 2007. Methyl salicylate is a critical mobile signal for plant systemic acquired resistance. Science 318: 113–116. [DOI] [PubMed] [Google Scholar]
- Patkar RN, Benke PI, Qu Z, Chen YY, Yang F, Swarup S, Naqvi NI. 2015. A fungal monooxygenase-derived jasmonate attenuates host innate immunity. Nature Chemical Biology 11: 733–740. [DOI] [PubMed] [Google Scholar]
- Qian B, Su X, Ye Z, Liu X, Liu M, Shen D, Chen H, Zhang H. 2022. MoErv29 promotes apoplastic effector secretion contributing to virulence of the rice blast fungus Magnaporthe oryzae. New Phytologist 233: 1289–1302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spence CA, Lakshmanan V, Donofrio N, Bais HP. 2015. Crucial roles of abscisic acid biogenesis in virulence of rice blast fungus Magnaporthe oryzae. Frontiers in Plant Science 6: 1082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spoel SH, Dong X. 2008. Making sense of hormone crosstalk during plant immune responses. Cell Host & Microbe 3: 348–351. [DOI] [PubMed] [Google Scholar]
- Wang J, Du Y, Zhang H, Zhou C, Qi Z, Zheng X, Wang P, Zhang Z. 2013. The actin-regulating kinase homologue MoArk1 plays a pleiotropic function in Magnaporthe oryzae. Molecular Plant Pathology 14: 470–482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang R, Ning Y, Shi X, He F, Zhang C, Fan J, Jiang N, Zhang YU, Zhang T, Hu Y et al. 2016. Immunity to rice blast disease by suppression of effector-triggered necrosis. Current Biology 26: 2399–2411. [DOI] [PubMed] [Google Scholar]
- Wang Y, Wang Y. 2018. Trick or treat: microbial pathogens evolved apoplastic effectors modulating plant susceptibility to infection. Molecular Plant–Microbe Interactions 31: 6–12. [DOI] [PubMed] [Google Scholar]
- Wilson RA, Talbot NJ. 2009. Under pressure: investigating the biology of plant infection by Magnaporthe oryzae. Nature Reviews Microbiology 7: 185–195. [DOI] [PubMed] [Google Scholar]
- Yang C, Li W, Cao J, Meng F, Yu Y, Huang J, Jiang L, Liu M, Zhang Z, Chen X et al. 2017. Activation of ethylene signaling pathways enhances disease resistance by regulating ROS and phytoalexin production in rice. The Plant Journal 89: 338–353. [DOI] [PubMed] [Google Scholar]
- Yang B, Wang Y, Guo B, Jing M, Zhou H, Li Y, Wang H, Huang J, Wang Y, Ye W et al. 2019a. The Phytophthora sojae RXLR effector Avh238 destabilizes soybean Type2 GmACSs to suppress ethylene biosynthesis and promote infection. New Phytologist 222: 425–437. [DOI] [PubMed] [Google Scholar]
- Yang C, Yu Y, Huang J, Meng F, Pang J, Zhao Q, Islam A, Xu N, Tian Y, Liu J. 2019b. Binding of the Magnaporthe oryzae chitinase MoChia1 by a rice tetratricopeptide repeat protein allows free chitin to trigger immune responses. Plant Cell 31: 172–188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang DL, Yang Y, He Z. 2013. Roles of plant hormones and their interplay in rice immunity. Molecular Plant 6: 675–685. [DOI] [PubMed] [Google Scholar]
- Yin Z, Chen C, Yang J, Feng W, Liu X, Zuo R, Wang J, Yang L, Zhong K, Gao C et al. 2019a. Histone acetyltransferase MoHat1 acetylates autophagy-related proteins MoAtg3 and MoAtg9 to orchestrate functional appressorium formation and pathogenicity in Magnaporthe oryzae. Autophagy 15: 1234–1257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin Z, Feng W, Chen C, Xu J, Li Y, Yang L, Wang J, Liu X, Wang W, Gao C et al. 2019b. Shedding light on autophagy coordinating with cell wall integrity signaling to govern pathogenicity of Magnaporthe oryzae. Autophagy 16: 900–916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan M, Ngou BPM, Ding P, Xin XF. 2021. PTI-ETI crosstalk: an integrative view of plant immunity. Current Opinion in Plant Biology 62: 102030. [DOI] [PubMed] [Google Scholar]
- Zhang H, Tang W, Liu K, Huang Q, Zhang X, Yan X, Chen Y, Wang J, Qi Z, Wang Z et al. 2011. Eight RGS and RGS-like proteins orchestrate growth, differentiation, and pathogenicity of Magnaporthe oryzae. PLoS Pathogens 7: e1002450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H, Zheng X, Zhang Z. 2016. The Magnaporthe grisea species complex and plant pathogenesis. Molecular Plant Pathology 17: 796–804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang S, Yang L, Li L, Zhong K, Wang W, Liu M, Li Y, Liu X, Yu R, He J et al. 2019. System-wide characterization of MoArf GTPase family proteins and adaptor protein MoGga1 involved in the development and pathogenicity of Magnaporthe oryzae. mBio 10: e02398–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou JM, Zhang Y. 2020. Plant immunity: danger perception and signaling. Cell 181: 978–989. [DOI] [PubMed] [Google Scholar]
- Zhu X, Zhou T, Chen L, Zheng S, Chen S, Zhang D, Li G, Wang Z. 2016.Arf6 controls endocytosis and polarity during asexual development of Magnaporthe oryzae. FEMS Microbiology Letters 363: fnw248. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig. S1 Southern blot analysis of the Moiug4 mutant.
Fig. S2 Localization of MoIug4 fused with NLS during infection in rice cells.
Fig. S3 Localization of MoIug4 expressed in Nicotiana benthamiana.
Fig. S4 MoIug4 is important for the suppression of ethylene production in rice during infection by Magnaporthe oryzae.
Fig. S5 Enriched sequences from the Chip-Seq analysis were exhibited.
Fig. S6 MoIgu4 specifically binds with the OsEIN2 promoter.
Fig. S7 Putative MoIug4-interacting proteins identified by screening the rice cDNA library.
Fig. S8 Controls for the pull-down and BIFC assays.
Fig. S9 Validation of transgenic lines.
Fig. S10 OsAHL1 is important for the rice resistance to Magnaporthe oryzae.
Fig. S11 The OsAHL1 N-terminal domain binds with the OsEIN2 promoter.
Fig. S12 MoIug4 and OsAHL1 are important for ethylene production and ethylene signaling transduction during interaction between rice and Magnaporthe oryzae.
Fig. S13 MoIug4 showed no effects on the protein stability of OsAHL1.
Fig. S14 MoIug4 showed no effects on the protein stability of OsAHL1.
Fig. S15 Activity of OsAHL1 in yeast.
Fig. S16 Localization and function of MoIug4 in OsAHL1-KO lines.
Table S1 Identification of MoIug4 in Guy11.
Table S2 Information of ChIP-seq analysis of MoIug4.
Data Availability Statement
The data that support the findings of this study are available in the Supporting Information of this article.


