Table 1.
Optimization of the reaction conditionsa
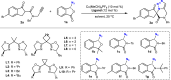 | ||||||
|---|---|---|---|---|---|---|
| Entry | L | “N3” | Solvent | Time (h) | Yield (%) | ee (%) |
| 1 | L1 | 1a | DCE | 24 | 35 | 14 |
| 2 | L2 | 1a | DCE | 24 | 28 | 12 |
| 3 | L3 | 1a | DCE | 24 | 50 | 23 |
| 4 | L4 | 1a | DCE | 24 | 37 | 75 |
| 5 | L5 | 1a | DCE | 24 | 44 | 44 |
| 6 | L6 | 1a | DCE | 24 | 40 | 82 |
| 7 | L7 | 1a | DCE | 24 | 45 | 80 |
| 8 | L8 | 1a | DCE | 24 | 62 | 92 |
| 9 | L9 | 1a | DCE | 24 | 47 | 33 |
| 10 | L10 | 1a | DCE | 24 | 52 | 31 |
| 11 | L8 | 1a | DCM | 24 | 70 | 94 |
| 12b | L8 | 1b | DCM | 24 | 30 | 27 |
| 13c | L8 | 1c | DCM | 48 | 80 | 90 |
| 14c | L8 | 1d | DCM | 48 | 99 | 94 |
| 15c | L8 | 1e | DCM | 48 | 78 | 94 |
| 16c | L8 | 1f | DCM | 48 | 95 | 93 |
| 17c | L8 | 1g | DCM | 48 | 93 | 93 |
DCE: 1,2-dichloroethane, DCM: dichloromethane, L: Ligand; N3: high iodine azide reagent; 1a: azidobenziodoxolone, 1b: azidodimethylbenziodoxole; 1c–g: azidobenziodazolones.
aReaction conditions: 2a (0.1 mmol), 1a (0.15 mmol), Cu(MeCN)4PF6 (10 mol%), L (12 mol %), solvent (2 mL), 25 °C; isolated yields; ee values were determined by HPLC analysis.
b1b was used in place of 1a.
cThe designated azidating reagent was used in place of 1a; 5 mol % of Cu(MeCN)4PF6 and 6 mol% of L8 were used instead.
