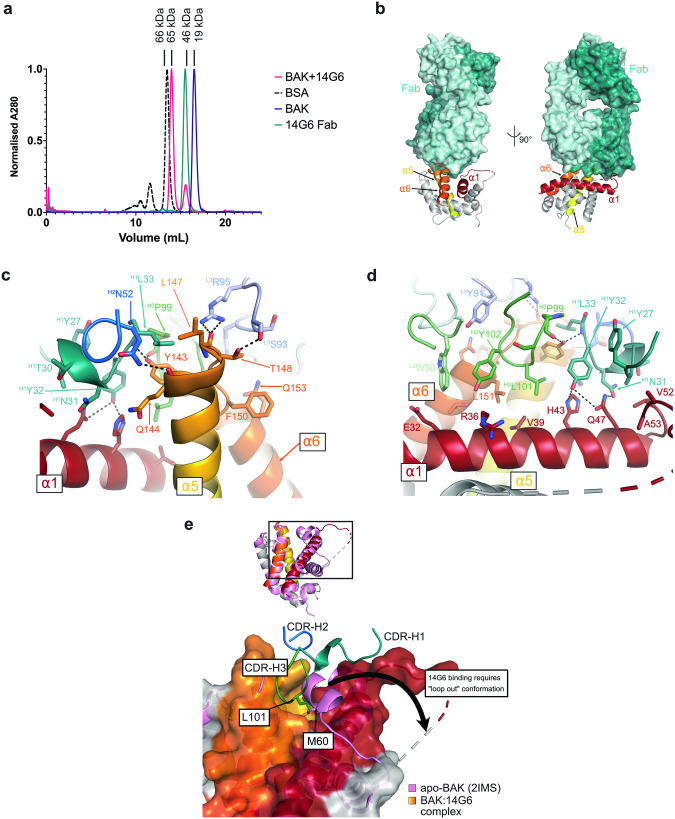
Fig. 3. Crystal structure of 14G6-bound human BAK reveals mechanism of inhibition.
a Size exclusion chromatography demonstrates that 14G6 Fab forms a 1:1 complex with BAK. BSA, bovine serum albumin, MW 66 kDa. b 14G6 binds BAK via interactions with the α5-α6 hinge (α5 and α6 shown in yellow and orange, respectively) and α1 (shown in red). The light and heavy chains of the Fab are shown in light and dark teal, respectively. c. Detailed interactions between 14G6 and BAK α5-α6 hinge. The light chain complementarity determining regions (CDRs) are shown in pale cyan (L1), light green (L2) and light blue (L3), and the heavy chain CDRs are shown in teal (H1), blue (H2), green (H3). d Detailed interactions between 14G6 and BAK α1. Colours as per (c). e 14G6 binding displaces the α1-α2 loop and inserts CDR-H3 L101 into the M60 pocket. apo-BAK (2IMS, pink) with closed α1-α2 loop overlaid with 14G6-bound BAK (grey, yellow, orange, red, chain D) showing open, unresolved loop.