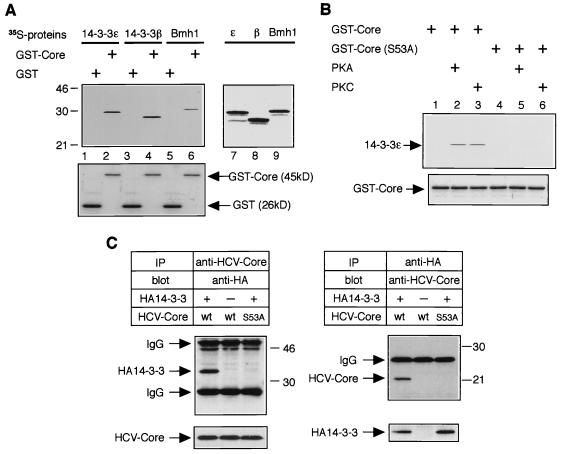
FIG. 2.
Interaction of HCV core protein with 14-3-3 protein in vitro and in mammalian cells. (A) HCV core protein interacts with several 14-3-3 protein isoforms in vitro. Three isoforms of 35S-labeled 14-3-3 proteins were subjected to a GST pull-down experiment using budding yeast-produced GST-Core protein (top panel, lanes 2, 4, and 6) and GST alone (top panel, lanes 1, 3, and 5). Positions of molecular weight standards (in kilodaltons) are shown at the left. Input of 35S-labeled 14-3-3 proteins (14-3-3ɛ, 14-3-3β, and Bmh1) are shown in lanes 7, 8, and 9, respectively. Coomassie blue-stained GST and GST-Core from the same gel are aligned to show protein content (bottom panel, lanes 1 to 6). (B) Phosphorylation of serine-53 by PKA or PKC is essential for HCV core–14-3-3 interaction in vitro. Bacterially produced GST-Core proteins (wild type and the S53A mutant) were treated with or without recombinant PKA or recombinant PKC and subjected to GST pull-down experiments using 35S-labeled 14-3-3ɛ protein. (C) HCV core protein interacts with 14-3-3 protein in mammalian cells. HepΔNCTH cells expressing wild-type (wt) HCV core protein and HepΔNCTH cells expressing the S53A mutant of HCV core protein (S53A) were transiently transfected with pCAHA-14-3-3ɛ [HA14-3-3(+)] or pCAHA empty vector [HA14-3-3(−)]. After 48 h, cell lysate was subjected to immunoprecipitation (IP) and Western blot (blot) with the antibodies indicated above the upper panels. The presence of HCV core protein and HA14-3-3 protein in extracts was verified by reprobing the same membranes with antibodies for immunoprecipitation (bottom lanes). Positions of molecular mass standards (in kilodaltons) are shown at the right.