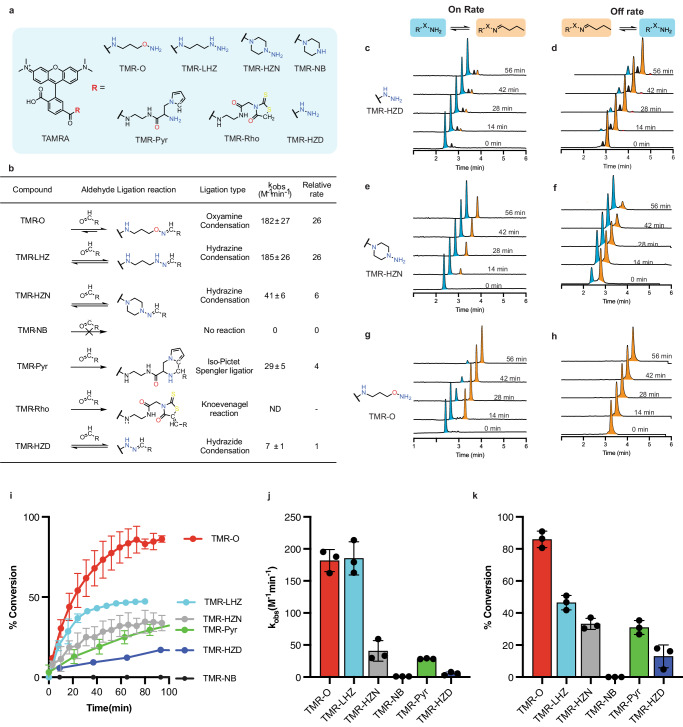
Fig. 1. Reactivity of a library of aldehyde-binding fluorescent probes.
a Chemical structure of the synthesized TAMRA-based probes. b Aldehyde-ligation reaction types and corresponding reaction rate constants relative to the commercially available TMR-HZD. The rate constant of the oxyamine probe (TMR-O) is 27-fold higher than TMR-HZD. c–h Reaction rates of the probes with butyraldehyde were followed by HPLC with fluorescence detection. HPLC traces for the forward (c, e, g) and reverse (d, f, h) reactions for TMR-HZD, TMR-HZN and TMR-O with butyraldehyde showing the ligation product (orange) and the free, unbound probe (blue). Of note, the HPLC traces for the commercially available TMR-HZD (c, d) also contain a small unidentified impurity (black). i Plots of the observed rate constant for the formation of the aldehyde-ligation products, (j) the corresponding percent conversion as a function of time and, (k) the percent product generated at 100 min. The non-binding control probe (TMR-NB), in which a cyclic piperazine group replaces the reactive hydrazine, shows no reactivity, confirming the specificity of the oxyamine (TMR-O), hydrazine (TMR-HZN) and pyrrole (TMR-Pyr) based TAMRA probes for aldehydes. TMR-O, however, has the fastest reactivity and highest percent conversion to ligation product. Values are plotted as the average of three experiments ± standard deviation. Source data are provided in the Source Data file.