Figure 6.
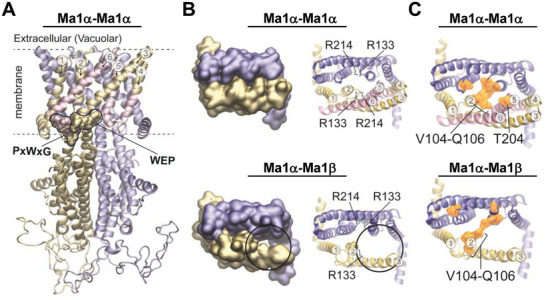
Homology modeling of the Ma1 protein structures. A) Comparative model of the Ma1α homodimer with monomers visualized in yellow and blue, respectively. Transmembrane domains missing in Ma1β are highlighted in pink. Two conserved PxWxG and WEP motifs are highlighted in the Surface representation. Yellow residues of the motifs are presented in Ma1α and Ma1β, pink residues are only presented in Ma1α. Six helices of the membrane‐spanning N‐terminal domain of the protein are numbered. Dashed lines indicate the position of the membrane. B) Top views of only the membrane‐spanning region with each monomer in a different color (blue or yellow). Represented are the Ma1α homodimer (top) and the Ma1α‐Ma1β heterodimer (bottom). On the right, arginine residues with importance for malate binding are highlighted. Four arginine residues are present in the Ma1α homodimer and three in the Ma1α‐Ma1β heterodimer. C) Top views of only the membrane‐spanning region in NewCartoon representation. Residues that are equivalent to those contributing to the extracellular gate in AtALMT1 are highlighted in orange Surface representation. In the Ma1α homodimer, V104, S105, Q106, and T204 are highlighted in each monomer. In the Ma1α‐Ma1β heterodimer, T204 is missing in Ma1β (yellow): V104, S105, and Q106 versus Ma1α (blue): V104, S105, Q106, and T204.
