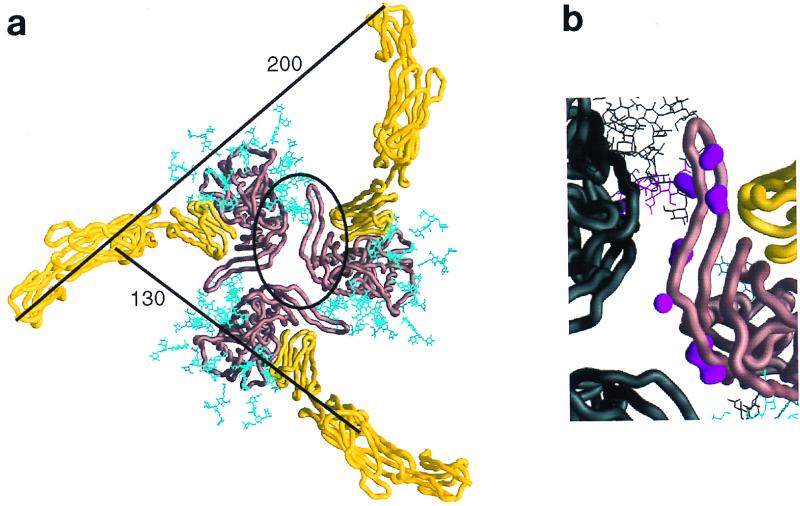
FIG. 7.
Selected features of the oligomeric gp120 model. (a) The entire extracellular portion of CD4 (yellow Cα worm) is shown binding to the oriented gp120 oligomer (copper-brown Cα worm). Carbohydrate on the gp120 is colored cyan. Distances between adjacent CD4 molecules are shown for the Cα of residue 178 at the end of the second domain and the Cα of residue 363, the last ordered residues prior to the transmembrane-spanning region. The contact region highlighted in panel b has been circled. The hemagglutinin proportionate model is used here, and thus the gp120 is close to the minimum protomer packing distance. (b) An enlargement of the contact site between gp120 core protomers in the oligomer. The coloring for one protomer is the same as in panel a; the other protomers are colored black. In addition, the molecular surfaces of side chains of conserved exposed residues in this contacting region (residues 195 to 210) are colored magenta. Starting from the top of the figure, these include residues 196, 197, 198, 201, 204, 208, and 209. Residue 197 is glycosylated, and its carbohydrate has been depicted in magenta. This region is sensitive to the binding of CD4, which is seen to the right of the figure in close proximity. The orientation of both images is shown from the perspective of the target cell membrane.