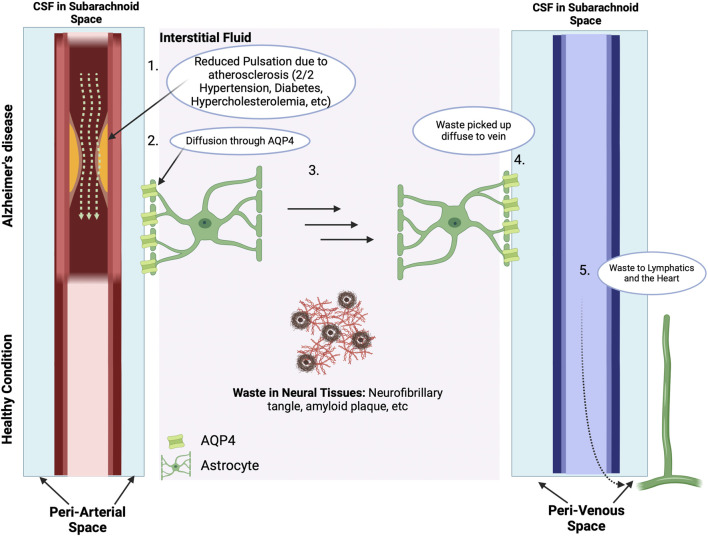
FIGURE 7.
The Vascular Hypothesis. The Blood-Brain Barrier and ApoE4: There are many waste products in the interstitial space, including Aβ and tau proteins and neurofibril tangles. With normal cerebral vascular function and pulsatile blood flow, the direction of CSF flow is from the periarterial space to the interstitial space to perivenous space. While the interstitial fluid crosses the AQP4 channel on the astrocytes of the perivenous space, the waste products in the interstitial space get carried along to the perivalvular space and to the lymphatic system. However, for subjects with vascular abnormalities, such as due to atherosclerosis, the decreased pulsatile blood flow drives CSF flow from the periarterial to interstitial to perivenous space, thus resulting in accumulation of beta amyloid and tau protein in the interstitial space. The presence of amyloid plaques in turn causes additional vascular dysfunction by damaging nearby blood vessels and disrupting the regulation of blood flow in the brain (Thomas et al., 1996). Subjects who carry the ApoE4 genotype have a reduced ability to clear Aβ, which then accumulates in brain microvessels and parenchyma (Martel et al., 1997), and is then associated with reduced cerebral blood flow and metabolism across multiple cortical regions, increasing the risk of hypoxic brain injury (Small et al., 1995; Mielke et al., 1998; Kim et al., 2013). In addition, cognitively normal ApoE4 carriers show significant age-related deficits in cerebral perfusion as they age, which increases the risk of AD (Thambisetty et al., 2010; Liu et al., 2013). Carrying the ApoE4 also heightens the risk of pericyte dysfunction thereby reducing the critical role of pericytes in maintaining the integrity of the blood brain barrier (BBB) thereby allowing Aβ and other inflammatory molecules to penetrate the CNS, inducing neuroinflammation and accelerating AD in part via inhibition of the anti-inflammatory effects of the TREM2-DAP12 complex on microglia (Armulik et al., 2010; Nishitsuji et al., 2011; Halliday et al., 2016; Fitz et al., 2021; Iannucci et al., 2021; Zhou et al., 2023). Data from ApoE4 carriers shows that the breakdown in the BBB starts in the medial temporal lobe, which is the part of the brain critical for cognitive function (Montagne et al., 2020). In consequence, inflammation and oxidative stress associated with both vascular dysfunction and Aβ worsen the damage caused by each factor, creating a feedback loop that accelerates AD progression. This figure was created with BioRender.com.