Figure 1.
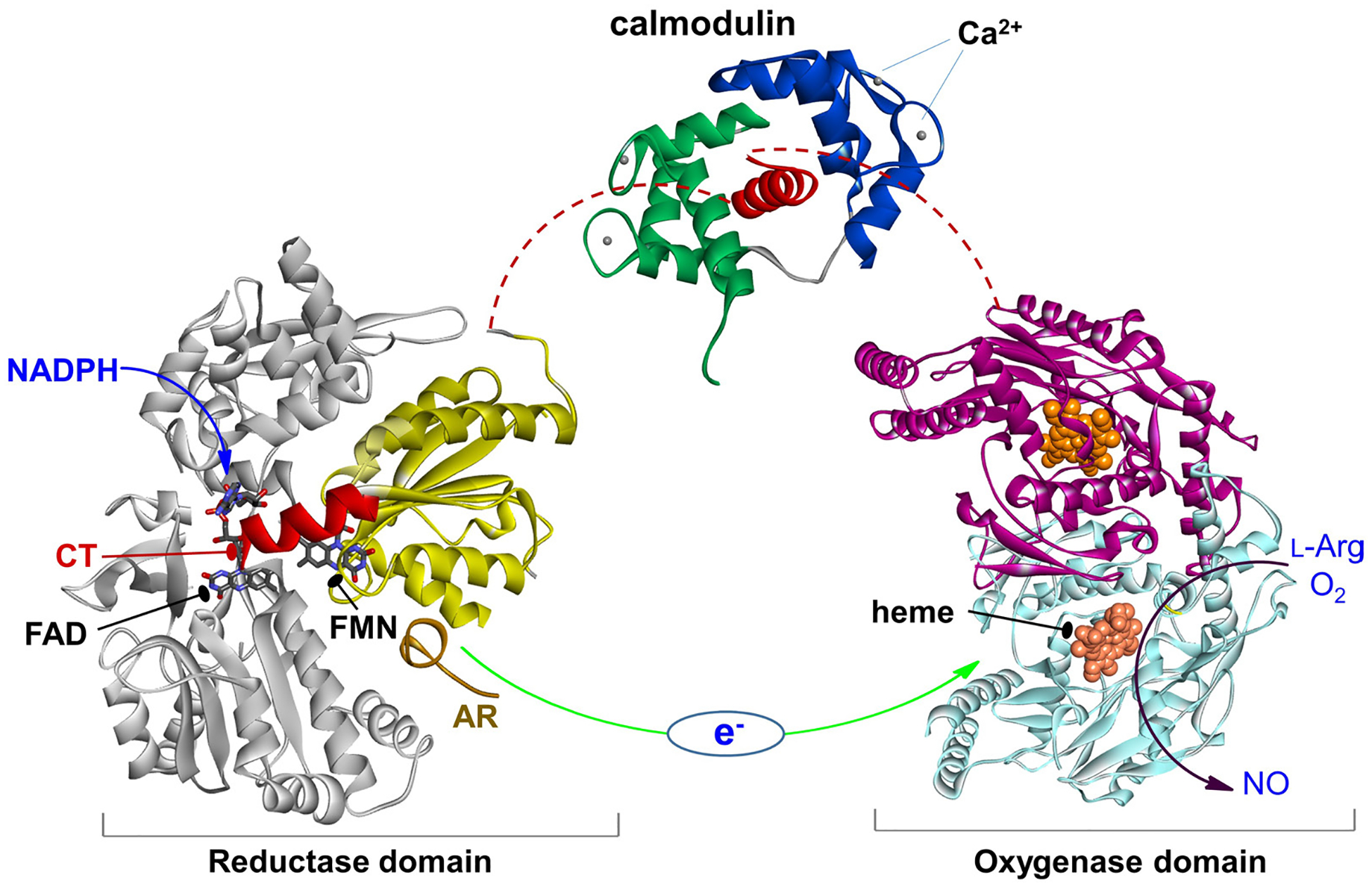
The NOS domains/modules: reductase domain with the FMN (sub)domain colored in yellow and the rest in gray, an a helical CaM-binding linker with bound CaM, and dimeric heme domain. Only the individual domain structures are available to date. CaM binding to NOS enables the electron transfer between the FMN and heme domains (green arrow). This involves a conformational change, in which the FMN domain shuttles between the FAD and heme domains to transfer the NADPH-derived electrons.
