Figure 3.
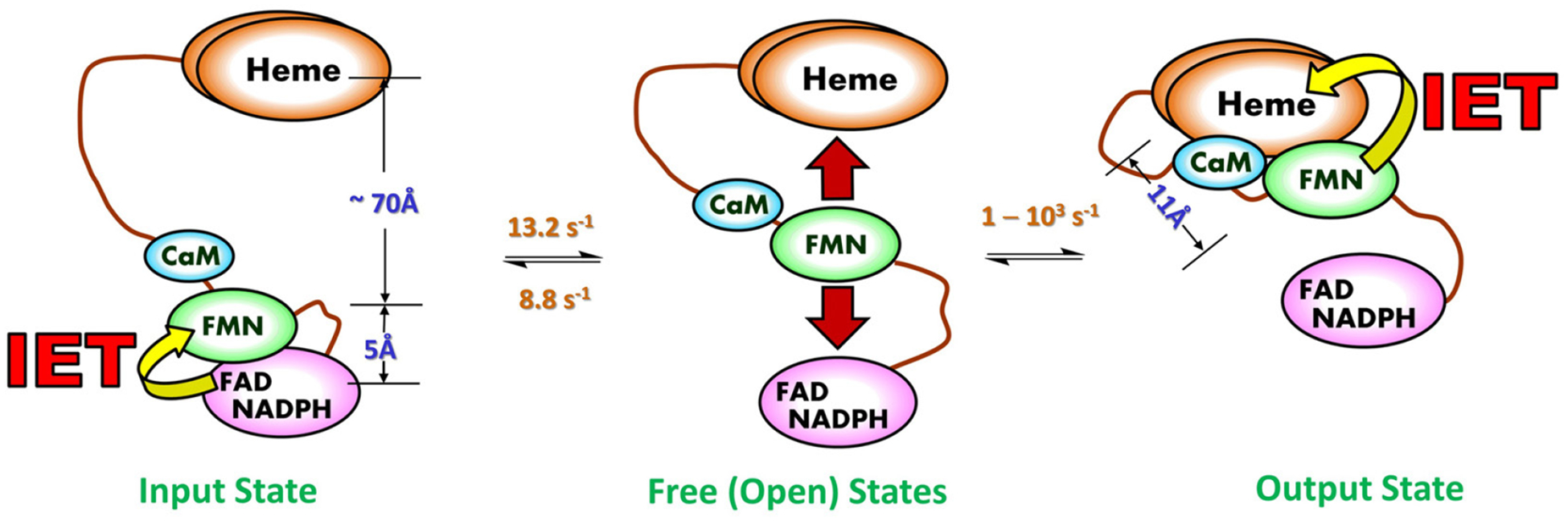
Tethered shuttle model (with the tethers corresponding to the interdomain FAD-FMN and FMN-heme linkers). This involves motion of the FMN domain between electron-accepting “input state” and electron-donating “output state”. The intermediate undocked free/open state(s) between the terminal docking positions is characterized by a continuum of conformations and exists in equilibrium with the docked conformations of the input and output states. The IET related edge-to-edge distances between redox cofactors (FAD, FMN and heme) in a rat nNOS input state model are displayed in blue, along with the edge-to-edge distance between FMN and heme in an iNOS output state model. The interchange rates between the NOS states are also displayed; the rates are of the CaM-bound eNOS isoform, which are currently available in the literature (63, 64). The conformational rates are at best semi-quantitative, and the docked/undocked rates of the interconversion between the free and output states can only be estimated within a large range (two to three orders of magnitude) (63). It is of note that the docked FMN/heme complex is transient (58), indicating that the docked and undocked rates differ significantly. As the three NOS isozymes show different turnover numbers, the isoform should have distinct conformational rates and distance.
