Abstract
Objective:
To assess acceptability and feasibility of rapid at-home COVID-19 testing and reporting of test results among individuals seeking care at community health centers (CHCs) and their household members.
Methods:
Participants were recruited from 2 Community Health Centers during a clinic visit or a community event. Over-the-counter COVID-19 tests were distributed to participants for self-testing and to offer testing to household members. Separate surveys were administered to collect baseline information on the study participant and to collect test results on the study participant and household members. We calculated the proportion of individuals who agreed to complete COVID home testing, those who reported test results, and the test positivity. For household members, we calculated the proportion who completed and reported results and the positivity rate. We assessed reasons for undergoing COVID-19 testing and the action taken by participants who reported positive tests.
Results:
A total of 2189 individuals were approached by CHC staff for participation and 1013 (46.3%) agreed to participate. Among the 959 participants with complete sociodemographic data, 88% were Hispanic and 82.6% were female. The proportion providing test results was 36.2% and the test positivity was 4.2%. Among the 1927 test reports, 35.3% for the index participant and 64.4% were for household members. The largest proportion of test results were for index participants (35.3%) and the second largest was for the participant’s children (32.1%), followed by parents (16.9%), and spouse/partner (13.2%). The 2 most common reasons for testing were symptoms (29%) and attending family gatherings (26%). Among test-positive individuals (n = 80), most (83.3%) noted that they isolated but only 16.3% called their provider and 1.3% visited a clinic.
Conclusion:
Our results show interest in at-home COVID-19 testing of multiple household members, as we headed into the endemic phase of the pandemic. However, reporting of test results was modest and among test-positive individuals, reporting results to a provider was very low. These results underscore the challenges with reporting and following guidelines among people undergoing home testing for COVID-19, which may have implications for future pandemics.
Keywords: community health, COVID, health inequities, health promotion, underserved communities
Background
There are pronounced inequities and disparities in morbidity and mortality from the 2019 novel coronavirus disease (COVID-19) reported among Black, Hispanic, and Native American communities in the United States (U.S.).1,2 These are largely due to comorbid conditions, factors related to social determinants of health, and structural racism. 3 Reduction in COVID-19 transmission can be accomplished by improving ventilation standards and masking of infected individuals for source protection, in addition to actions at the population level such as vaccination and access to rapid COVID-19 testing,4,5 which includes home testing. Effective implementation of recommended public health regimens could help limit disease spread and reduce the need for more costly measures associated with caring for moderate and severe COVID-19 disease. Community Health Centers (CHCs), which serve predominantly low-income and racially/ethnically diverse patients,6,7 can be important clinical and public health allies in reaching these populations. Through their community outreach events, CHCs can quickly distribute home tests for entire households and promote early detection. When combined with government supported early treatment programs (eg, Paxlovid or Lagevrio for early onset COVID-19) low-income persons would have timely access to treatments and avoid severe illness.
As we move away from the public health emergency phase of the COVID-19 pandemic, interest in testing has wavered. Lessons learned from both the emergency and endemic phases of the pandemic are critical in the event of the emergence of variants of concern and of future pandemics, which will likely include home testing. Questions remain regarding the acceptability of at-home testing, particularly of individual’s willingness to report results, given that there are no mandates for this reporting. 8 Some literature has highlighted individual’s willingness to use at-home tests9,10; however, willingness and actual testing are different entities. In addition, among low-income families, especially those who have multiple household members, it is important to assess individual’s willingness to offer testing to all household members and report their test results. These “household” behaviors may be as important as individual COVID-19 mitigation behaviors such as mask-wearing, social distancing, and hand hygiene. 11 Further, little is known about the implementation of effective public health measures, such as isolation, following positive COVID-19 tests, nor whether individuals contact their health provider or health center to be assessed for anti-viral therapy.
The objective of this study was to assess the acceptability and feasibility of rapid at-home COVID-19 testing and reporting of test results among individuals seeking care at CHCs, which are designated as Federally Qualified Health Centers (FQHCs). We also assessed the feasibility and acceptability of study participants offering testing to their household members and reporting their test results.
Methods
Recruitment and Eligibility
The COVID CoNOce MÁS/COVID KNOW MORE study was conducted in partnership with 2 CHCs in San Diego County, the fifth largest U.S. County by population. Study enrollment was conducted from June 25, 2022, to April 30, 2023, before the Centers for Disease Control and Prevention (CDC) declared the end of the pandemic phase (May 11, 2023). 12 Patients aged 21 years and older were eligible to enroll during a clinic visit that occurred for any reason, or via attendance at a community event sponsored by the CHC (ie, a clinic mobile unit or health fair). Participants were recruited by CHC clinic staff during a patient’s clinic visit or attendance at community events. Study participants (index participant) provided written informed consent agreeing to fill out a survey, allow access to limited medical record data, complete self-collected at-home testing, and report test results through a secure Research Electronic Data Capture (REDCap) survey. They were provided with over-the-counter COVID-19 tests (ie, Flowflex) equal to 2 tests per household member for up to 5 household members, including themselves (10 total tests). Participants were asked to have household members complete at-home testing and provide their test results via a Quick Response (QR) code App. Household members included individuals older than 2 years of age who reside in the home address of the participant. A waiver of informed consent was obtained for household members, whose test results were provided by the index participant and did not include personally identifiable information.
The Flowflex over-the-counter home test was used for testing. The test is Food and Drug Administration (FDA) authorized for nonprescription home use with self-collected (unobserved) direct anterior nasal swab specimens. Index participants were provided with an educational handout and instructions that described potential situations when they and eligible household members should consider testing, including: (1) COVID-19 symptoms; (2) travel; (3) family gatherings; (4) contact with infected individual; and (5) contact with someone at high risk (such as older individual and immune compromised individual). Participants were encouraged to test more than once as these scenarios arose, or anytime they were worried about COVID-19 infection. Directions from the test kit manufacturer were reviewed with index participants during enrollment.
Data Collection and Statistical Analysis
Data collection
We developed 2 online surveys for data collection. We collected and stored all survey data via the REDCap platform. REDCap is a secure and Health Insurance Portability and Accountability Act (HIPAA)-compliant web-based application for building and managing online surveys and databases. The first survey was an enrollment questionnaire, which was completed by the index participant at the time of enrollment at the participating health center or community event. The second survey was a test result questionnaire. This was completed by index participants to report their test results, and/or the results for household members, either using a QR code or via a web link provided by the health center at the time of consent (on each test kit). The test result survey was completed each time a participant or a household member completed a COVID-19 home test. The QR code and online link to the test survey can be accessed via Supplemental Materials.
The following self-reported data were collected from each index participant using the enrollment survey: age, sex, race and ethnicity, access to health services, income, employment status, education, English proficiency, food insecurity, gender identity, health insurance, COVID-19 illness history, COVID-19 vaccination status, experience, and barriers of past COVID-19 testing and knowledge and attitude to COVID-19 testing and vaccination. All of these variables were consider relevant to the interpretation of our results for at-home COVID-19 testing among diverse household members. We also collected data on household size, foreign-born status, and job type, given that these factors contribute to higher infection rates in underserved communities,1,13,14 as well as trust in healthcare and perception of discrimination. In the test result survey, we collected data specific to home testing about the individual completing the test (index participant or household member), including: (1) age, sex, race and ethnicity, and relationship to the index participant, (2) if multiple household members tested at the same time, (3) date of testing of each individual, (4) reason for testing, (5) testing results, and (6) actions taken if tested positive.
Statistical analysis
We described the demographic and clinic COVID-related test results and vaccination. among index participants by clinic site. For index participants, we calculated the proportion of individuals who agreed to complete COVID home testing and to report test results, as well as the proportion of participants who completed at least 1 test, provided test results, and test positivity. For household members, we calculated the proportion who completed at least 1 test and reported results, as well as the positivity rate for submitted test results. We reported reasons for undergoing COVID-19 testing and the action taken by participants who reported positive tests. We compared index participants who submitted at least 1 COVID-19 test result with those who did not submit results using t-tests (for continuous variables) and Chi-square tests (for categorical variables).
Results
Characteristics of Index Participants
During the recruitment period (between June 25, 2022, to April 30, 2023), a total of 2189 individuals were approached by CHC staff to solicit participation (824 in CHC1 and 1694 in CHC2). Of these, 1013 participants were recruited (510 at CHC1 and 503 at CHC2) resulting in an enrollment rate of 61% in CHC1 and 29% in CHC2. CHC1 recruited participants through clinic sites and a few local vaccine events whereas CHC2 recruited in local events and resource fairs. The median (min-max) household size was 4 (1-10) residents. Per our research protocol, we only provided test kits for up to 5 household members with 2 tests per kit. The median number of test kits distributed per index participant was 8. After exclusion of observations with missing data for age (n = 39), sex (n = 2), and race and ethnicity (n = 13), 959 index participants were available for analysis. Table 1 shows the characteristics of the final study population of index participants. The mean age of the participants was 42.9 years (SD = 13.3) and the majority were women (82.6%). The population was majority Hispanic (88%), with most born outside the United States (63%). More than one half of participants (52%) were married and 35% had less than a high school education. In terms of health insurance coverage, 43% had public insurance (Medicare, Medicaid, Tricare), 27% had private insurance, and 22% were uninsured. A large proportion reported being vaccinated (89%) and 80% reported having received a booster vaccine.
Table 1.
Characteristics of Index Participants.
| CHC1 (N = 476) | CHC2 (N = 483) | Total (N = 959) | |
|---|---|---|---|
| Age mean (SD) | 40.4 (13.1) | 45.3 (13.1) | 42.9 (13.3) |
| Female | 387 (81.3%) | 405 (83.9%) | 792 (82.6%) |
| Marital status | |||
| Never been married | 117 (24.6%) | 80 (16.7%) | 197 (20.7%) |
| Married | 228 (48%) | 266 (55.5%) | 494 (51.8%) |
| Divorced, widowed, or separated | 72 (15.2%) | 80 (16.7%) | 152 (15.9%) |
| A member of an unmarried couple | 27 (5.7%) | 33 (6.9%) | 60 (6.3%) |
| Prefer not to answer | 31 (6.5%) | 20 (4.2%) | 51 (5.4%) |
| Education | |||
| Have never gone to school | 18 (3.8%) | 22 (4.5%) | 40 (4.2%) |
| Less than high school | 119 (25.2%) | 216(44.7%) | 335 (35.1%) |
| High school graduate or GED completed | 109 (23.1%) | 92 (19.1%) | 201 (21.1%) |
| Some college/technical/vocational degree | 134 (28.4%) | 75 (15.5%) | 209 (21.9%) |
| Associate of Arts (AA) Degree | 19 (4.0%) | 13 (2.7%) | 32 (3.4%) |
| Bachelor’s or advanced degree | 59 (12.5%) | 58 (12.0%) | 117 (12.3%) |
| Prefer not to answer or don’t know | 14 (3.0%) | 7 (1.5%) | 21 (2.2%) |
| Born outside United States | 235 (51.7%) | 356 (74.2%) | 591 (63.2%) |
| Race/ethnicity | |||
| Hispanic | 403 (84.7%) | 439 (90.9%) | 842 (87.8%) |
| Non-Hispanic white | 35 (7.4%) | 24 (5.0%) | 59 (6.2%) |
| Black, excludes Hispanic | 12 (2.5%) | 6 (1.2%) | 18 (1.9%) |
| Asian, excludes Hispanic | 7 (1.5%) | 6 (1.2%) | 13 (1.4%) |
| NH/PI, excludes Hispanic | 2 (0.4%) | 0 | 2 (0.2%) |
| AI/AN, excludes Hispanic | 5 (1.1%) | 0 | 5 (0.5%) |
| Multiracial, excludes Hispanic | 3 (0.6%) | 4 (0.8%) | 7 (0.7%) |
| Health Insurance | |||
| No health insurance | 81 (17.1%) | 127 (26.4%) | 208 (21.8%) |
| Private | 155 (32.7%) | 103 (21.4%) | 258 (27.0%) |
| Public (Medicare, Medicaid, and Tricare) | 191 (40.3%) | 222 (46.1%) | 413 (43.2%) |
| Don’t know | 15 (3.2%) | 9 (1.9%) | 24 (2.5%) |
| Prefer not to answer | 32 (6.8%) | 21 (4.4%) | 53 (5.5%) |
| Prior positive COVID-19 test | 315 (66.2%) | 263 (54.6%) | 578 (60.3%) |
| Received a COVID vaccine | 425 (89.5%) | 426 (88.9%) | 851 (89.2%) |
| Received a COVID-19 vaccine booster | 358 (85.4%) | 314 (74.1%) | 672 (79.7%) |
Test Reporting
The proportion of index participants reporting test results was 36.2% (367/1013). Among the 1013 index participants, 7858 test kits were distributed and of these, 1927 (25%) test reports were submitted (Table 2). The proportion of tests reported was 27% in CHC 1 and 22% in CHC 2. The proportion of test positivity was 3.2% in CHC1 and 5.4% in CHC2, with an overall 4.2% positivity (ie, the total number positive tests (80) divided by the total number of reported tests (1927)). Among the 1927 test reports, 676 (35.3%) were completed by the index participant and the remaining 1241 (64.4%) were completed by household members. The median number of test results reported by index participants for their household was 4 (range 1-10); 20.4% submitted only 1 test result. As shown in Figure 1, the largest proportion of test reports were for the index participants (35.3%), followed by reports for their children (32.1%). Parents of the index participants represented 16.9% of the reported testing and 13.2% were for the spouse/partner. Smaller proportions of test results were for the participants’ siblings (6.1%) and grandchildren (1.7%).
Table 2.
COVID-19 At-home Test Uptake and Test Results Submitted.
| CHC | No. participants | No. test kits distributed | No. test results submitted (%) | No. reported positive tests (%) |
|---|---|---|---|---|
| 1 | 510 | 4096 | 1112 (27.1) | 36 (3.2) |
| 2 | 503 | 3762 | 815 (21.7) | 44 (5.4) |
| Total | 1013 | 7858 | 1927 (24.5) | 80 (4.2) |
Figure 1.
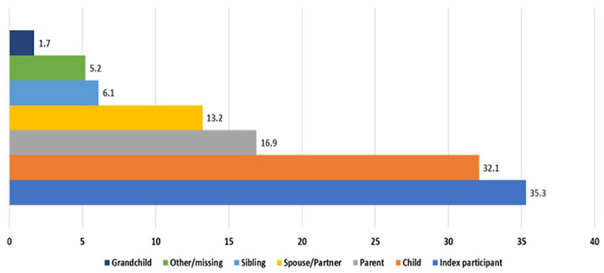
Reporting of COVID-19 Test Results for Household Members.
Table 3 presents a comparison of characteristics of index participants who submitted a test result versus those who did not. Compared to individuals who submitted test results, those who did not submit a result were significantly older (P < .05), and more likely to be male (P < .05). The proportion of patients from CHC1 who submitted results was higher than that of CHC2 (P < .05). There were no significant differences in result submission by Hispanic ethnicity, health insurance status, or having been vaccinated.
Table 3.
Comparison of Index Participants Who Submitted at Least 1 COVID-19 Test Result With Those Who Did Not Submit Results.
| Yes (N = 351) | No (N = 662) | |
|---|---|---|
| Age, mean (SD)* | 38.9 (12.2) | 45.0 (13.4) |
| Female* | 296 (86.5%) | 534 (81.3%) |
| Health center* | ||
| CHC1 | 199 (56.7%) | 311 (47.0%) |
| CHC2 | 152 (43.3%) | 351 (53.0%) |
| Hispanic/Latino | 295 (84.3%) | 579 (87.6%) |
| Health insurance a | 256 (78.3%) | 448 (74.7%) |
| Received a COVID vaccine | 319 (91.7%) | 578 (87.7%) |
Including private (purchased directly or through employment) and public (Medicare, Medi-Cal, and Tricare).
P < .05.
Reasons for Testing
We ascertained reasons for testing (Table 4). The most common reason for testing was due to symptoms (29%). Other reasons included family gatherings (26%), contact with an infected individual (18%), to protect other high-risk individuals from getting infected (17%), and travel (10%). Smaller proportions were related to school or work requirements, other large gatherings/events and testing to get clearance from a positive test.
Table 4.
Reported Reasons for COVID-19 at-Home Testing.
| Reasons | CHC1 (N = 1112) (%) | CHC2 (N = 815) (%) | Total (N = 1927) (%) |
|---|---|---|---|
| Symptoms | 368 (33.1) | 187 (23.0) | 555 (28.8) |
| Family gathering | 264 (23.7) | 230 (28.2) | 494 (25.6) |
| Contact with infected individual | 163 (14.7) | 180 (22.1 ) | 343 (17.8) |
| Protect others at high-risk for COVID-19 | 211 (19.0) | 125 (15.3) | 336 (17.4) |
| Travel | 121 (10.9) | 80 (9.8) | 201 (10.4 ) |
| School or work requirement | 9 (0.8 ) | 23 (2.8) | 32 (1.7) |
| Other large gathering or events | 4 (0.4 ) | 16 (2.0) | 20 (1.0) |
| Tested to get clearance from a positive test | 0 | 4 (0.5) | 4 (0.2) |
Actions Taken Among Participants Testing Positive
Individuals who tested positive were asked what actions they took based on CDC recommendations (Table 5). Respondents could select more than 1 response. Of the 80 individuals who tested positive, 4 (5%) did not indicate an action. Most said that they isolated (67, 83.8%); thirteen (16.3%) respondents noted that they called their CHC and 1 additional individual (1.3%) had a clinic visit.
Table 5.
Actions Among Participants Who Reported Positive Tests (N = 80 Participants).
| Action taken if tested positive | N = 130 responses a (%) |
|---|---|
| Undergoing isolation | 67 (83.8) |
| Calling the clinic | 13 (16.3) |
| In-person clinic visit | 1 (1.3) |
| Missing work or school | 49 (61.3) |
Responses were not mutually exclusive, resulting in a higher number of responses than participants.
Discussion
We report on the uptake of COVID-19 at-home testing and test results among patients from 2 large CHCs who were predominantly of Hispanic ethnicity (88%). Test results also included participants’ household members. Our results show continued interest in testing as the U.S. headed into the endemic phase of the public health emergency. However, reporting of test results via an easy-to-use mobile app was modest (~36%). Results underscored study participant’s willingness to have their household members tested, with the participant’s children being the second most common members for whom testing was reported, after the index participant. This is important given that living in crowded households is a predictor of COVID-19 infection.1,14
Data on feasibility and acceptability of COVID-19 at-home testing among medically underserved communities, including CHC patients, are scarce. Data on willingness to provide results from home testing in this vulnerable population are also lacking. From a public health perspective, at-home rapid testing offers accessibility without the need for an in-person visit. 15 This addresses common clinic visit barriers such as work leave and transportation. 16 With results being available within 15 to 20 min, at-home testing permits early detection of positive cases, facilitating reduction of subsequent COVID-19 transmission. This is particularly important for individuals without primary healthcare and other medically underserved community members. Conversely, rapid testing poses some challenges, given that this relies on the individual’s willingness to self-test and to report positive results to public health officials or to the individual’s physician. Furthermore, home-based testing foregoes the need for providers to offer follow up care, including re-testing. Our results show that among participants who tested, reporting of results was modest. It is important to note that in our study, this reporting pertains to results for the index participant and all household members who tested. Younger individuals and female participants were more likely to provide results than older or male participants.
While the 36% of participants who reported testing was modest, we could argue that by the end of the pandemic phase, most people, including those served by CHCs, had acquired the skills necessary to mitigate transmission in the household and to engage in care when they really needed it, including medications.17,18 It is also plausible that the modest reporting was because no additional steps were needed to know what to do to mitigate transmission and to seek care if their clinical status deteriorated.
Our results show that among CHC patients who reported test results, the most common reason for testing was due to symptoms (30%), which is consistent with data from a nation-wide U.S. survey where 29% of the respondents also noted symptoms as their primary reason for at-home testing. 15 Visiting a family member or attending family gatherings were also top reasons in the same U.S. survey (17%) and in our study (26%). This suggests that among our low-income, predominantly Hispanic study population, which is not representative of the rest of the country, the major reasons for completing at-home testing are similar to those of the larger U.S. population.
We further show that among participants who reported a positive test, most (84%) said they practiced isolation, which helps reduce the spread of the virus. However, only 16% reported following up with a health care provider. 19 Although COVID-19 self-test instructions recommend that users report results to their health care provider for additional testing and treatment, published data show that individuals who self-test are less likely to report results and to follow these instructions than those who are tested by a provider, even during the time of the public health emergency.15,20 This is concerning, especially given that most of the index participants have a medical home at the participating CHC (ie, 72% of index participants were CHC patients). This low percentage represents a more contemporary behavior, reflective of a timeline closer to the end of the public health emergency. During the survey period, June 2022 to April 2023, sublineages of the Omicron variant continued to circulate 21 but these tended to cause less severe disease compared to previous variants, and this was widely reported in the media. Also, in March of 2022 the Biden-Harris Administration nationwide “Test-to-Treat” initiative was launched. 22 The initiative attempts to mitigate severe COVID-19 illness, by providing quick access to treatments such as Paxlovid or Lagevrio with an emphasis on underserved population. The “Home Test to Treat Program” is specifically for uninsured, Medicare, Medicaid, VA insurance, and/or Indian Health Service patients. Related to our findings, 84% of participants with a COVID positive test that did not contact a health care provider would not or did not avail themselves of these programs.
Limitations of our study relate to the fact that responses were self-reported and thus, we were not able to confirm the test results. Also, there is the potential for self-selection bias from those participants who decided to enroll in the study versus those that did not. Furthermore, we only collected detailed data via the enrollment survey on index participants and not among the household members. The study participants included CHC patients and a high proportion of women. Thus, these results are not generalizable to the U.S. population. Given the changing pace of the pandemic, the results of home-based testing and reporting by the study participants might not reflect the true practice in the U.S. over time. Specifically, participation and reporting took place during the latter part of pandemic phase, which ended on May 11, 2023. 12 It is also possible that results would have been different if reporting took place during peak pandemic periods.
Conclusion
The COVID-19 pandemic provided an unprecedented opportunity for the U.S. to produce tools and resources to better prepare for the next public health emergency. Our results show interest in at-home COVID-19 testing of multiple household members, as we headed into the endemic phase of the pandemic. However, reporting of test results was modest and among test-positive individuals, reporting results to a provider was very low. These results underscore the challenges with reporting and following guidelines among people undergoing home testing for COVID-19, which may have implications for future pandemics.
Supplemental Material
Supplemental material, sj-docx-1-jpc-10.1177_21501319241259684 for Distribution of COVID-19 Home Testing Through Community Health Centers: Results of the COVID CoNOce MÁS Study by Jesse N. Nodora, Maria Elena Martinez, Corinne McDaniels-Davidson, Jian Shen, Amy M. Sitapati, Francesca Torriani, Jess Mandel and Linda Hill in Journal of Primary Care & Community Health
Footnotes
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This research was funded under NIH/NCI award UH3CA233314.
ORCID iD: Jesse N. Nodora  https://orcid.org/0000-0001-6756-4423
https://orcid.org/0000-0001-6756-4423
Supplemental Material: Supplemental material for this article is available online.
References
- 1. Oh DL, Meltzer D, Wang K, et al. Neighborhood factors associated with COVID-19 cases in California. J Racial Ethn Health Disparities. 2023;10(6):2653-2662. doi: 10.1007/s40615-022-01443-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Webb Hooper M, Nápoles AM, Pérez-Stable EJ. COVID-19 and racial/ethnic disparities. JAMA. 2020;323(24):2466-2467. doi: 10.1001/jama.2020.8598 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Truman BI, Chang MH, Moonesinghe R. Provisional COVID-19 age-adjusted death rates, by race and ethnicity - United States, 2020-2021. MMWR Morb Mortal Wkly Rep. 29 2022;71(17):601-605. doi: 10.15585/mmwr.mm7117e2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Centers for Disease Control and Prevention. COVID-19 testing overview. August 25, 2020. Accessed January 4, 2024. https://www.cdc.gov/coronavirus/2019-ncov/cases-updates/testing-in-us.html
- 5. Oran DP, Topol EJ. Prevalence of asymptomatic SARS-CoV-2 infection : a narrative review. Ann Intern Med. 2020;173(5):362-367. doi: 10.7326/M20-3012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Health Resources and Services Administration Bureau of Primary Health Care. What is a Health Center? 2023. Accessed January 3, 2024. https://bphc.hrsa.gov/about-health-centers/what-health-center
- 7. National Association of Community Health Centers. America’s Health Centers: By the Numbers. Updated August 7, 2023. Accessed January 4, 2024. https://www.nachc.org/resource/americas-health-centers-by-the-numbers/
- 8. Rubin R. COVID-19 testing moves out of the clinic and into the home. JAMA. 2021;326(14):1362-1364. doi: 10.1001/jama.2021.15679 [DOI] [PubMed] [Google Scholar]
- 9. Goggolidou P, Hodges-Mameletzis I, Purewal S, Karakoula A, Warr T. Self-testing as an invaluable tool in fighting the COVID-19 pandemic. J Prim Care Community Health. 2021;12:21501327211047782. doi: 10.1177/21501327211047782 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Kiene SM, McDaniels-Davidson C, Lin CD, et al. At-home versus onsite COVID-19 school-based testing: a randomized noninferiority trial. Pediatrics. 2023;152(1):e2022060352F. doi: 10.1542/peds.2022-060352F [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Sanchez T, Hall E, Siegler AJ, et al. Prevalence of COVID-19 mitigation behaviors in US adults (August-December 2020): nationwide household probability survey. JMIR Public Health Surveill. 2023;9:e37102. doi: 10.2196/37102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Centers for Disease Control and Prevention. End of the federal COVID-19 public health emergency (PHE) declaration. Updated September 12, 2023. Accessed January 2, 2024. https://www.cdc.gov/coronavirus/2019-ncov/your-health/end-of-phe.html
- 13. Figueroa JF, Wadhera RK, Lee D, Yeh RW, Sommers BD. Community-level factors associated with racial and ethnic disparities in COVID-19 rates in Massachusetts. Health Aff (Millwood). 2020;39(11):1984-1992. doi: 10.1377/hlthaff.2020.01040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Martinez ME, Nodora JN, Carvajal-Carmona LG. The dual pandemic of COVID-19 and systemic inequities in US Latino communities. Cancer. 2021;127(10):1548-1550. doi: 10.1002/cncr.33401 [DOI] [PubMed] [Google Scholar]
- 15. Ritchey MD, Rosenblum HG, Del Guercio K, et al. COVID-19 self-test data: challenges and opportunities - United States, October 31, 2021-June 11, 2022. MMWR Morb Mortal Wkly Rep. 2022;71(32):1005-1010. doi: 10.15585/mmwr.mm7132a1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Crane JT, Fabi R, Pacia D, Neuhaus CP, Berlinger N. “We’re here to take care of our community”: lessons learned from the U.S. Federal Health Center covid-19 vaccine program. Health Promot Pract. 2024;25(1):137-144. doi: 10.1177/15248399221151178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Quandt SA, LaMonto NJ, Mora DC, Talton JW, Laurienti PJ, Arcury TA. COVID-19 pandemic among Latinx farmworker and nonfarmworker families in North Carolina: knowledge, risk perceptions, and preventive behaviors. Int J Environ Res Public Health. 2020;17(16):5786. doi: 10.3390/ijerph17165786 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Mehta SN, Burger ZC, Meyers-Pantele SA, et al. Knowledge, attitude, practices, and vaccine hesitancy among the Latinx community in southern California early in the COVID-19 pandemic: cross-sectional survey. JMIR Form Res. 2022;6(8):e38351. doi: 10.2196/38351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. D’Agostino EM, Rosenberg LM, Richmond A, et al. You & me: test and treat study protocol for promoting COVID-19 test and treatment access to underserved populations. BMC Public Health. 2023;23(1):2121. doi: 10.1186/s12889-023-16960-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Moonan PK, Smith JP, Borah BF, et al. Home-based testing and COVID-19 isolation recommendations, United States. Emerg Infect Dis. 2023;29(9):1921-1924. doi: 10.3201/eid2909.230494 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. World Health Organization. Statement on Omicron sublineage BA.2. 2022. Accessed January 2, 2024. https://www.who.int/news/item/22-02-2022-statement-on-omicron-sublineage-ba.2
- 22. Department of Health and Human Services Administration for Strategic Preparedness and Response. Test to Treat. 2024. Accessed January 2, 2024. https://aspr.hhs.gov/TestToTreat/Pages/default.aspx
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-docx-1-jpc-10.1177_21501319241259684 for Distribution of COVID-19 Home Testing Through Community Health Centers: Results of the COVID CoNOce MÁS Study by Jesse N. Nodora, Maria Elena Martinez, Corinne McDaniels-Davidson, Jian Shen, Amy M. Sitapati, Francesca Torriani, Jess Mandel and Linda Hill in Journal of Primary Care & Community Health


