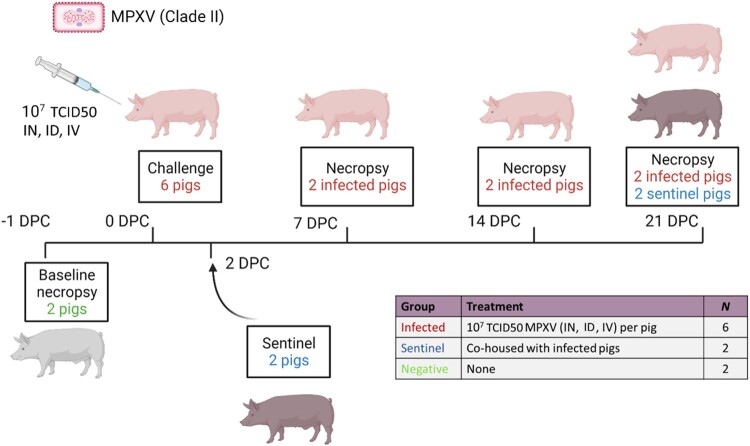
Figure 1.
Experimental design. Six pigs were inoculated with the MPXV hMPXV/USA/MA001/2022 (Lineage B.1, Clade IIb) isolate acquired from BEI Resources. A 3 ml dose of 3 × 107 TCID50 per animal was administered intranasal (IN), intradermal (ID), and intravenous (IV). At 2 days post-challenge (DPC), two contact sentinel control pigs were co-mingled with the six principally challenged animals. Daily clinical observations and body temperatures were recorded. Nasal, oropharyngeal, and rectal swabs as well as whole blood were collected at −1, 1, 3, 5, 7, 10, 14, 17, and 21 DPC. Oral fluids and serum were collected at −1, 7, 14, and 21 DPC. Post-mortem examinations were performed at 7, 14, and 21 DPC and results compared to baseline post-mortem examinations conducted on 2 additional negative control pigs at -1 DPC. BioRender.com was used to create the figure illustrations.