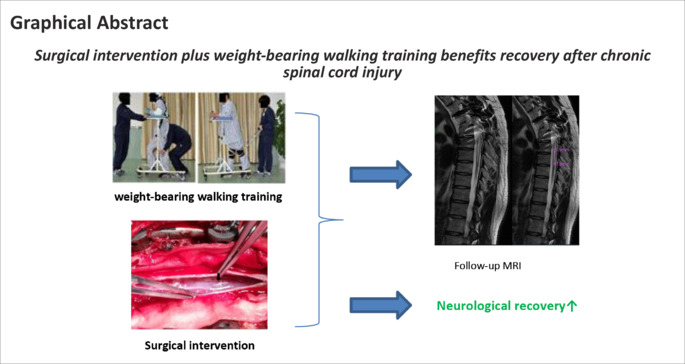
Keywords: chronic spinal cord injury, intensive rehabilitation, locomotor training, neurological recovery, surgical intervention, weight-bearing walking training
Abstract
For patients with chronic spinal cord injury, the conventional treatment is rehabilitation and treatment of spinal cord injury complications such as urinary tract infection, pressure sores, osteoporosis, and deep vein thrombosis. Surgery is rarely performed on spinal cord injury in the chronic phase, and few treatments have been proven effective in chronic spinal cord injury patients. Development of effective therapies for chronic spinal cord injury patients is needed. We conducted a randomized controlled clinical trial in patients with chronic complete thoracic spinal cord injury to compare intensive rehabilitation (weight-bearing walking training) alone with surgical intervention plus intensive rehabilitation. This clinical trial was registered at ClinicalTrials.gov (NCT02663310). The goal of surgical intervention was spinal cord detethering, restoration of cerebrospinal fluid flow, and elimination of residual spinal cord compression. We found that surgical intervention plus weight-bearing walking training was associated with a higher incidence of American Spinal Injury Association Impairment Scale improvement, reduced spasticity, and more rapid bowel and bladder functional recovery than weight-bearing walking training alone. Overall, the surgical procedures and intensive rehabilitation were safe. American Spinal Injury Association Impairment Scale improvement was more common in T7–T11 injuries than in T2–T6 injuries. Surgery combined with rehabilitation appears to have a role in treatment of chronic spinal cord injury patients.
Introduction
Spinal cord injury (SCI) is a devastating medical condition with far-reaching consequences for individuals and their families. SCI substantially burdens healthcare systems and economies through lost productivity and extremely high life-long healthcare costs (Badhiwala et al., 2019; Malekzadeh et al., 2022). The overall estimated global incidence of SCI is approximately 1298 cases per million (Li et al., 2023). Although significant progress has been made in understanding SCI’s cellular and molecular mechanisms, treatment and management protocols aimed at ameliorating neurologic damage remain largely ineffective. Most neuroprotective therapies have focused on treating patients in the acute or subacute period, but over 90% of patients with SCI are in the chronic phase (No authors listed, 2016). For patients with chronic SCI (CSCI), the conventional treatment is rehabilitation. In addition to rehabilitation, treating SCI complications is another focus (for example, urinary tract infections, pressure sores, osteoporosis, and deep vein thrombosis). Medications for improving SCI recovery have yet to be developed and surgeons seldom perform surgery on CSCI. The need for effective therapies in patients with CSCI is urgent.
In CSCI, the acute inflammatory response has subsided, fibrosis or glial scars have developed, and central cavitations have formed (Ditunno and Formal, 1994; Young, 2014; O’Donovan, 2016; Holmes, 2017; Martin 2022). Our previous research studied the evolution of SCI pathology in Macaca fascicularis, a non-human primate (Shi et al., 2009). Compared with pathology in rodents (Liu et al., 2006, 2007; Walker et al., 2012), we found delayed Wallerian degeneration, less astrocytic glial scar formation, and increased fibrotic scar in the macaques (Wu et al., 2013). Based on our experience, SCI still progresses steadily in the chronic phase. Fibrotic connective tissue invades the subarachnoid space and can tether the spinal cord. Focal tethering can block the flow of cerebrospinal fluid (CSF), increase local CSF tissue pressure, reduce spinal cord blood flow, and cause development of compressive subarachnoid cysts. In addition, posttraumatic syringomyelia may develop in some patients and expand over several spinal cord segments. Consequently, it is reasonable to presume that surgical detethering in the chronic phase could improve spinal cord function and protect the remaining cord tissue from further degeneration.
To date, few trials have focused on surgical intervention in the chronic phase of SCI, and most were retrospective case series rather than prospective randomized controlled trials (RCTs) (Falci et al., 2009; Stenimahitis et al., 2022). We hypothesize that surgical decompression or untethering of the injured spinal cord in the chronic phase of injury will restore CSF circulation, blood flow, and vascular pulsation and protect the remaining cord tissue from further degeneration. Rehabilitation is a routine treatment for CSCI. We further hypothesize that surgical intervention combined with implementation of the Kunming Locomotor Training Program (KLTP), a unique weight-bearing rehabilitation training program developed by our team (Zhu et al., 2016; Liu et al., 2021), will facilitate reorganization of the remaining neural circuitry and promote even greater locomotor, sensory and autonomic recovery. To examine these hypotheses, we performed a RCT in CSCI patients with a complete injury. Our aim was to determine the efficacy of combining surgical intervention with the weight-bearing walking training program.
Methods
Participants
Thirty patients (22 men and eight women) aged 18 to 60 years with a chronic (≥ 12 months since injury) complete traumatic SCI between the T1 and T12 segments were enrolled. All patients were admitted and treated at the Kunming Spine and Spinal Cord Injury Treatment Center at Kunming Tongren Hospital, Kunming, Yunnan, China between July 1, 2016 and June 30, 2018. Patients were informed in detail about the surgical procedure and rehabilitation plan. All provided informed consent for study participation. A signed written consent was also obtained from subjects for publication of their data, including images. The Science and Research Committee of Kunming Tongren Hospital approved all procedures. The study is reported in line with the 2010 Consolidated Standards of Reporting Trials guidelines (Schulz et al., 2010) and was conducted in accordance with the Declaration of Helsinki. The trial was registered at ClinicalTrials.gov (NCT02663310) on February 3, 2016.
All patients were neurologically examined on admission according to standards established by the American Spinal Injury Association (ASIA; Li et al., 2014) to confirm a complete SCI (ASIA Impairment Scale [AIS] grade A). Plain radiography and magnetic resonance imaging (MRI) of the spine were used to determine the site and severity of injury. Imaging was obtained during the screening visit and at the 12-month follow-up. One subject in the treatment group (enrollment number 01) received follow-up MRI 6 years after the initial screening. All subjects had prior spinal internal fixation surgery but none had previous surgery on the spinal cord. No subjects had participated any clinical trials or had other experimental treatment before enrollment. After enrollment, patients were randomly divided into treatment (surgery + rehabilitation) and control (rehabilitation only) groups. Each group comprised 15 patients (Figure 1).
Figure 1.
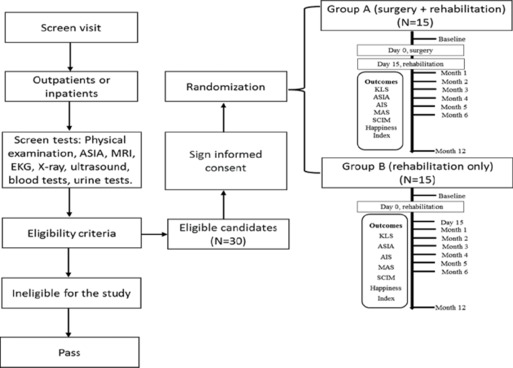
Study flow chart.
For randomization, 30 cards (15 labeled “A” and 15 labeled “B”) were folded and put into a box designed explicitly for randomization. Subjects were instructed to pick one card from the box, which was shown to a witness. “A” indicated treatment group assignment and “B” indicated control group. Selected cards were not returned to the randomization box.
Eligibility criteria
Inclusion criteria were as follows: 1) adult aged 18 to 60 years with a chronic traumatic SCI (≥ 12 months after SCI); 2) level of injury between T1 and T12; 3) AIS grade A injury; 4) MRI showed tethering adhesions; and 5) ability to read, write, and understand/use visual analog scales. We excluded: 1) patients with osteoporosis or significant joint disease; 2) patients with severe brain injury or other neurological disorder; 3) patients with active pressure sores, or known kidney, cardiovascular, or liver disorders; 4) patients with an infectious disease (including but not limited to hepatitis B and human immunodeficiency virus); 5) women who were pregnant or lactating; 6) patients with medical or mental instability according to the judgment of the investigator; 7) patients with a history of multiple sclerosis, transverse myelitis, or peripheral neuropathy; and 8) patients who, in the opinion of the investigator, would not be compliant with the study protocol.
Surgical intervention
Surgical intervention was performed only in the treatment group. The goal was to release tethering adhesions as completely as safely possible and to observe restoration of spinal pulsation and CSF flow. Both detethering and decompression were permitted. Preoperative MRI and intraoperative observations determined the location of spinal durotomy. After durotomy, the gross appearance of the spinal cord and sources of CSF flow interruption for each subject were examined under a surgical microscope to determine subsequent maneuvers. Generally, detethering was performed to free the spinal cord starting from the dorsal surface and proceeding laterally and ventrally on both sides. Both sharp dissection and careful blunt separation were used to free the spinal cord from pial and dural scarring adhesions. Spinal cord distraction or tension was minimized to avoid injury. If adhesions were suspected rostral or caudal to the window of dural exposure, a thin rubber urethral catheter was used to gently explore the space beneath the dura and separate adhesions if possible. The return of pulsatile spinal cord movement and evidence of CSF motion indicated satisfactory detethering. In patients with cystic degeneration of the spinal cord, myelotomy was performed using a small longitudinal incision over the site of degeneration and malacic necrotic tissue was removed. After the spinal cord portion of the operation was completed, dural closure with duraplasty was performed. Then, the paraspinous muscles, subcutaneous tissue, and skin were closed in layers. Postoperative care was initially provided in the intensive care unit until patients were medically stable for transfer to the general ward. The rehabilitation program was initiated 15 days after surgery.
Weight-bearing locomotor training
Subjects in the control group started weight-bearing ambulation training with supervision by training therapists after admission and evaluation. Treatment group subjects started 15 days after surgery. Training consisted of the KLTP (Liu et al., 2021), which comprises eight progressive steps as follows: 1) training the subject to stand supporting their own weight while a trainer fixes the knees, 2) training the subject to stand with his/her own weight support, 3) training the subject to walk with wheeled weight support while a trainer holds the knees or bandages attached to the subject’s knees during walking, 4) training the subject to make his/her own volitional steps with wheeled weight support, 5) training the subject to walk with the help of a non-wheeled four-leg support, 6) training the subject to walk with a pair of crutches, 7) training the subject to walk with a cane, and 8) training the subject to walk without any support (Figure 1). Subjects started at step 1 and then sequentially moved to the following ones gradually based on the trainer’s evaluation.
The program was carried out collectively in a single hall to allow maximal interaction, which generated an atmosphere of encouragement and inspiration from therapists and fellow subjects. Several simple tailored devices to promote ambulation, such as rubber bands to avoid foot drop, knee bending, and falling, were used so subjects could keep moving forward. KLTP training was carried out twice daily for 1.5 hours per session, 5 days per week for 235 days.
Urination and defecation training
KLTP multidisciplinary rehabilitation also includes urination and defecation training to mitigate urinary and fecal accidents. Urination management includes reflexive voiding and catheterization training; defecation management includes diet control and stimulation of defecation training.
Kunming Locomotor Scale
The Kunming Locomotor Scale (KLS; Liu et al., 2021) is a ten-grade locomotion scoring system adapted to the KLTP (Figure 2). Grade I refers to patients who can only sit in a wheelchair and cannot stand. Grades II to VIII refer to patients who can accomplish KLTP steps 1 to 7 consecutively. Grade IX represents patients who can walk without support (equivalent to KLTP step 8) but who show instability. Grade X represents a patient who can walk without support and without instability. Grade VI and above indicates that the patient does not need wheeled weight support for walking.
Figure 2.
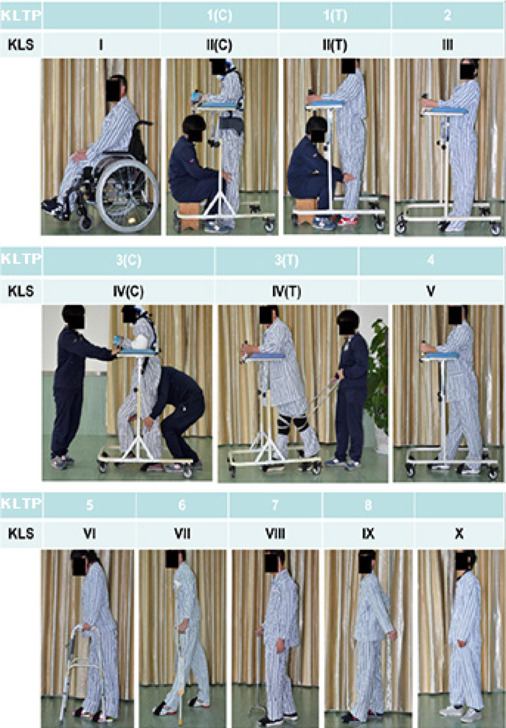
Kunming locomotor training program (KLTP) and Kunming Locomotor Scale (KLS) in patients with complete traumatic spinal cord injury.
The KLTP comprises eight progressive steps. All subjects were trained starting at step 1. Once the subject consistently completed step 1, the training moved on to the next step, and the training continued in this way through each subsequent step. The 10-point KLS was used to assess locomotor recovery. The KLS is indicated by Roman numerals I to X and is based on the KLTP.
KLS grade was evaluated in each patient and recorded at admission, 15 days after surgery (for patients in the treatment group), and 1, 2, 3, 4, 5, 6, and 12 months after rehabilitation began. Blinding was not possible as subjects who had surgery wore a spinal brace for 3 months.
Neurological evaluations
ASIA scores and AIS
Neurological deficits were defined according to the International Standards for Neurological Classification of Spinal Cord Injury (ISNCSCI; Rupp et al., 2021). ISNCSCI motor, pinprick, and light touch scores, AIS grade, and neurological level of injury (NLI) were evaluated by experienced neurologists and/or neurosurgeons and recorded at admission, 15 days after surgery (for subjects in the treatment group), and 1, 2, 3, 4, 5, 6, and 12 months after rehabilitation began. All evaluators attended the Training Course on Neurological Assessment of Spinal Cord Dysfunction (provided by the Department of Orthopedics and Traumatology at the University of Hong Kong Faculty of Medicine) and the Spinal Cord Outcome Assessment Workshop (provided by the China SCI Net and the University of Hong Kong Faculty of Medicine Spinal Cord Injury Fund).
Walking Index for Spinal Cord Injury II
The Walking Index for Spinal Cord Injury II (WISCI II) is a validated locomotor functional index that evaluates the degree of physical assistance and braces and devices a patient with SCI requires for walking (Dittuno and Ditunno, 2001; Ditunno et al., 2013). It is accepted as a measure of walking function for use in clinical trials. WISCI II score was evaluated and recorded at admission, 15 days after surgery (for subjects in the treatment group), and 1, 2, 3, 4, 5, 6, and 12 months after rehabilitation began.
Bladder and stool scores
We used the bladder and bowel sphincter management portions of the Spinal Cord Independence Measure (SCIM III) (Catz et al., 2007; Anderson et al., 2011) to assign a bladder score (BS) and stool score (SS). The BS consists of seven scale elements (scoring 0, 3, 6, 9, 11, 13, and 15) as follows: 0 points—indwelling catheter; 3 points—residual urine volume (RUV) > 100 mL, no regular catheterization or assisted intermittent catheterization; 6 points—RUV < 100 mL or intermittent self-catheterization, needs assistance to apply drainage instrument; 9 points—intermittent self-catheterization, uses external drainage instrument without assistance; 11 points—intermittent self-catheterization, continent between catheterizations, does not use external drainage instrument; 13 points—RUV < 100 mL, needs only external urine drainage, no assistance is required for drainage; 15 points—RUV < 100 mL, continent, does not use external drainage instrument.
The SS consists of four elements (scoring 0, 5, 8, and 10) as follows: 0 points—irregular timing or very low frequency (less than once in 3 days) of bowel movements; 5 points—regular timing, but requires assistance (e.g., for applying a suppository), rare accidents (less than twice a month); 8 points—regular bowel movements without assistance, rare accidents (less than twice a month); 10 points—regular bowel movements without assistance, no accidents.
BS and SS were evaluated and recorded at admission, 15 days after surgery (for subjects in the treatment group), and 1, 2, 3, 4, 5, 6, and 12 months after rehabilitation began.
Modified Ashworth Scale
The Modified Ashworth Scale (MAS) was used to measure spasticity in all subjects (Haas et al., 1996; Akpinar et al., 2017). MAS was evaluated and recorded at admission, 15 days after surgery (for subjects in the treatment group), and 1, 2, 3, 4, 5, 6, and 12 months after rehabilitation began.
Quality of life evaluations
Spinal Cord Independence Measure
The Spinal Cord Independence Measure (SCIM) was used to measure independence (Catz et al., 1997). SCIM was evaluated and recorded at admission, 15 days after surgery (for subjects in the treatment group), and 1, 2, 3, 4, 5, 6, and 12 months after rehabilitation began.
Happiness Index
The Happiness Index, designed by Zhu Hui’s team at Kunming Tongren Hospital, was used to measure psychological state. The Happiness Index consists of five items (1, 2, 3, 4, 5) as follows: 1 point—confined to the house and ashamed to see people, feels miserable, unable to take care of oneself; 2 points—able to take care of oneself; 3 points—able to take care of oneself and participate in outdoor activities; 4 points—able to start a business, economic independence; 5 points—sexual ability improved. Happiness Index was evaluated and recorded at admission, 15 days after surgery (for subjects in the treatment group), and 1, 2, 3, 4, 5, 6, and 12 months after rehabilitation began.
Statistical analysis
Because of the preliminary nature of this project, we used the highest feasible sample size within our budget constraints to cover patients’ medical expenses. A sample size of 30 (15 per group) allows detection of a large effect size of 1.06 standard deviations with 80% power using the two-sample t-test at a significance level of α = 0.05. Continuous and categorical variables were compared using the t-test and Fisher’s exact test, respectively. Mixed effect models were used to analyze repeated ISNCSCI, AIS, KLS, WISCI II, BS, SS, and MAS scores over the nine time points (admission, 15 days, and 1, 2, 3, 4, 5, 6, and 12 months) with separate scores as the dependent variable and time (as a categorical variable), randomization group, and interaction between time and randomization as fixed effects. Significant interactions between time and group would indicate differences in the change in scores from baseline between the two groups. A random subject effect was used in all models to allow for correlations from repeated scores for the same individuals over time. Post hoc Pearson correlation analysis was performed to assess the effect of NLI. Analyses were performed using SAS software version 9.3 (SAS Institute, Cary, NC, USA). The significance level was set to 0.05. General linear models were used to analyze repeated SCIM and Happiness Index scores over the nine-time points with scores of the time points as the within-subjects variable and randomization group as the between-subjects factor.
Results
Patient characteristics
All 30 subjects were classified as AIS grade A at baseline. The study population comprised 22 men (73.3%) and 8 women (26.7%). Mean age was 33 ± 6.4 years (range, 19–46). Mean time from injury to trial entry was 3.9 ± 3.9 years (Table 1). Although osteoporosis is common after SCI, no bone fractures or joint trauma were observed during the study. Rehabilitation using KLTP was safe and did not cause any serious complications. However, several surgical complications occurred in the treatment group. CSF leakage occurred in one subject which was treated using lumbar drainage, recumbent positioning, and 14 days of antibiotics (intravenous ceftriaxone once daily); surgical repair was not required. No life-threatening complications, organ impairment, loss of neurological function, or death occurred. Motor, sensory, and other neurological functions eventually improved among the subjects in the treatment group.
Table 1.
General admission data
| Variables | Overall (n=30) | Group A (n=15) | Group B (n=15) | P-value |
|---|---|---|---|---|
| Age at injury (yr) | 29.1±6.3 | 30.4±6.6 | 27.8±5.9 | 0.28 |
| Age at enrollment (yr) | 33.0±6.4 | 34.2±6.8 | 31.8±6.1 | 0.32 |
| Years since injury | 3.9±3.9 | 3.8±4.6 | 4.0±3.3 | 0.9 |
| Male (%) | 22 (73.3) | 12 (80) | 10 (66.7) | 0.68 |
Intraoperative conditions
The main intraoperative observations, associated surgical interventions, and surgical results are summarized in Table 2.
Table 2.
Intraoperative conditions observed, surgical procedures, and surgical results
| Intraoperative condition | n (%) | Surgical procedures | Results |
|---|---|---|---|
| Adhesion of the spinal arachnoid | 15 (100.0) | Separation of thicker scar adhesions and arachnoid bands using careful blunt separation or a sharp peeling method; release of arachnoid adhesions carefully with minimum traction. | Adhesions released |
| Blockage of cerebrospinal fluid flow | 15 (100.0) | Generally, the cerebrospinal fluid circulation will recover after the above treatment. If it does not recover, or the recovery is minimal, a small catheter is slowly and gently advanced around the rostral and caudal segments of the spinal cord | Cerebrospinal fluid flow restored |
| Absent pulsation of the spinal cord | 12 (80.0) | Spinal cord pulsation is restored after the complete release of the spinal cord from the dorsal, bilateral, and ventral tethering | Pulsation of the cord restored |
| Decreased venous plexus at spinal cord surface, and the spinal cord appeared pale | 11 (73.3) | Intraoperative condition cannot be surgically corrected at present | None |
| Arachnoid cysts | 5 (33.3) | After the cyst fluid was slowly extracted with a syringe, the cyst wall near the dura was partially removed | Cyst collapsed |
| Complete interruption of spinal cord continuity | 3 (20.0) | Intraoperative condition cannot be surgically corrected at present | None |
| Cystic degeneration of the spinal cord | 3 (20.0) | A small longitudinal incision (myelotomy) was made over the tissue of cystic degeneration region to remove intraspinal malacic necrotic tissue. | Necrotic tissues removed |
| Syringomyelia | 1 (6.7) | After separating adhesion and restoring normal cerebrospinal fluid circulation, the syrinx collapsed. | The volume of syringomyelia became smaller |
| Bone compression | 1 (6.7) | A vertebral canal exploration was applied to remove any compressive bone fragments. | Compression of bone on the spinal cord released |
| Spinal cord edema | 0 | None | None |
Neurological recovery
AIS and ASIA scores after intervention
Neurological function improved in both the treatment and control groups; however, some improvements were more extensive in the treatment group. At 12 months, the AIS grade improved to grade B in 7 subjects and grade C in 3 subjects of the treatment group; in contrast, only one control group patient improved to grade B (Figure 3). The proportion of patients who improved was significantly higher in the treatment group (66.7% vs. 6.7%; P = 0.0034).
Figure 3.
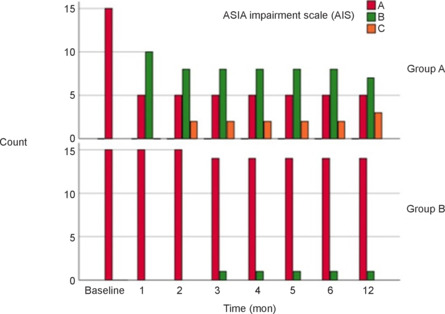
Change in the American Spinal Injury Association Impairment Scale (AIS) grade over time in both groups.
Group A: Treatment group (surgery + rehabilitation); group B: control (rehabilitation only) group.
In both groups, ISNCSCI motor score was slightly higher at 12 months than at baseline (P = 0.0045), however, the difference was not significant between the groups (P = 0.8552). Changes in ISNCSCI pinprick and touch sensory scores significantly differed between the groups over time (P = 0.009). In the treatment group, pinprick score was significantly higher at 12 months than at baseline; however, pinprick score was similar at these time points in the control group (Figure 4).
Figure 4.
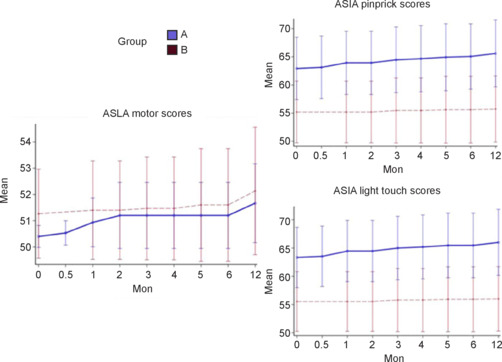
American Spinal Injury Association (ASIA) score with 95% confidence intervals according to group over time.
Group A: Treatment group (surgery + rehabilitation); group B: control (rehabilitation only) group.
Walking ability
Ambulatory function as measured by the KLS and WISCI improved quickly in both groups (Figure 5). There was a greater rate of score increase in the first month, followed by smaller but continued improvement in both groups. Surgical treatment had no apparent effect on either score (P = 0.25 for KLS and P = 0.30 for WISCI).
Figure 5.
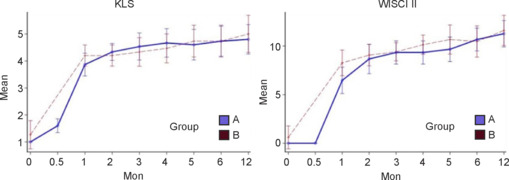
The Kunming Locomotor Scale (KLS) and Walking Index for Spinal Cord Injury II (WISCI II) scores with 95% confidence intervals according to group over time.
Group A: Treatment group (surgery + rehabilitation); group B: control (rehabilitation only) group.
Urination and defecation function
Urination and defecation improved over time in all subjects. Surgical detethering and decompression appeared to contribute to improvement in both functions. BS was significantly higher in the treatment group than the control group at 2 months (P = 0.0024) but not at 12 months (P = 0.43). In both groups, SS was significantly higher at 12 months than at baseline (P < 0.05). SS at 12 months was significantly higher in the treatment group (P < 0.05; Figure 6).
Figure 6.
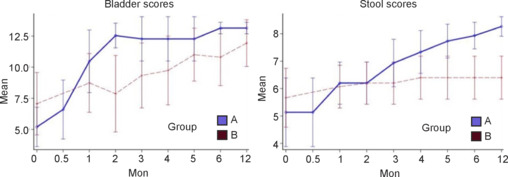
Bladder and stool scores with 95% confidence intervals according to group over time.
Group A: Treatment group (surgery + rehabilitation); group B: control (rehabilitation only) group.
Post-hoc correlation analysis
To determine if NLI influenced recovery, a post-hoc analysis was conducted to determine if there was a correlation between NLI and improvement in AIS grade in the treatment group. Interestingly, a significant correlation was found between lower NLI (T7-T11) and improvement from AIS grade A to grade B/C (Pearson’s r = 0.661, P = 0.038) However, there was no correlation between NLI and improvement in SS (Pearson’s r = −0.355, P = 0.314) or BS (Pearson’s r = 0.542, P = 0.105; Table 3).
Table 3.
Pearson correlation analysis to assess the effect of neurological level of injury on recovery
| ASI A to B/C (n) | NLI (n) | ||
|---|---|---|---|
| NLI | Pearson correlation | 0.661* | 1 |
| Sig. (two-tailed) | 0.038 | ||
| N | 10 | 10 | |
| ASI A to B/C (n) | Pearson correlation | 1 | 0.661* |
| Sig. (two-tailed) | 0.038 | ||
| N | 10 | 10 | |
| NLI | Stool score (improvement ≥ 3 to 10) (n) | ||
| NLI | Pearson correlation | 1 | –0.355 |
| Sig. (two-tailed) | 0.314 | ||
| N | 10 | 10 | |
| Bowel (improvement ≥ 3/to maximun of 10) | Pearson correlation | –0.355 | 1 |
| Sig. (two-tailed) | 0.314 | ||
| N | 10 | 10 | |
| NLI | Bladder score (improvement ≥ 5 to 15) (n) | ||
| NLI | Pearson correlation | 1 | 0.542 |
| Sig. (two-tailed) | 0.105 | ||
| N | 10 | 10 | |
| Bladder (improvement ≥ 5/to maximun of 15) | Pearson correlation | 0.542 | 1 |
| Sig. (two-tailed) | 0.105 | ||
| N | 10 | 10 |
ASI: American Spinal Injury Association Impairment Scale; NLI: neurological level of injury. *Correlation is significant (P < 0.05)
MAS score
In both groups, the MAS score was lower at 12 months than at baseline, indicating less spasticity. MAS score was significantly lower in the treatment group than the control group at 12 months (P = 0.04; Figure 7).
Figure 7.
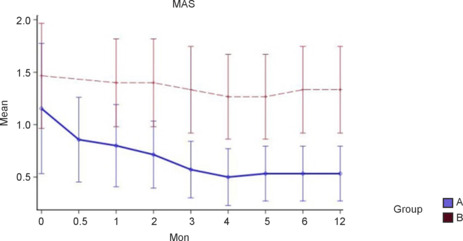
The Modified Ashworth Scale (MAS) score with 95% confidence intervals according to group over time.
Group A: Treatment group (surgery + rehabilitation); group B: control (rehabilitation only) group.
SCIM score
In both groups, the SCIM score was higher at 12 months than at baseline, indicating greater independence (P < 0.001). SCIM score was significantly higher in the treatment group at 12 months (P < 0.001; Figure 8).
Figure 8.
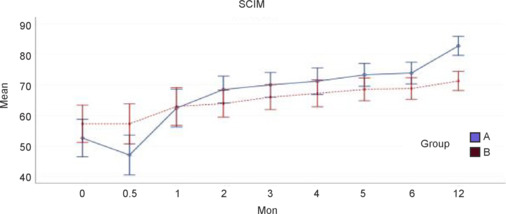
The Spinal Cord Independence Measure (SCIM) score with 95% confidence intervals according to group over time.
Group A: Treatment group (surgery + rehabilitation); group B: control (rehabilitation only) group.
Happiness Index
In both groups, the Happiness Index score was higher at 12 months than at baseline (P < 0.001); however, the scores did not significantly differ between the groups (Figure 9).
Figure 9.
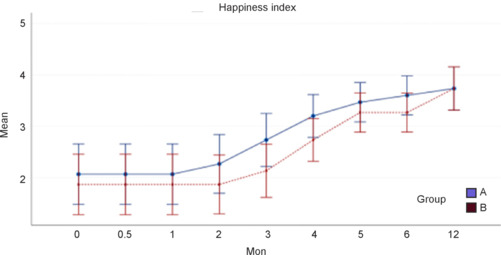
Happiness Index score with 95% confidence intervals according to group over time.
Group A: Treatment group (surgery + rehabilitation); group B: control (rehabilitation only) group.
Follow-up MRI
MRI of a treatment group patient is shown in Figure 10. Imaging before surgery shows a T4 level of injury, artifact from thoracic spinal instrumentation (from a previous spinal stabilization operation), dilation of the central canal of the spinal cord below the level of injury, and narrowing of the subarachnoid CSF space. Postoperative imaging at the 6-year follow-up shows considerable improvement in the central canal dilation and expansion of the subarachnoid CSF circulation pathway.
Figure 10.
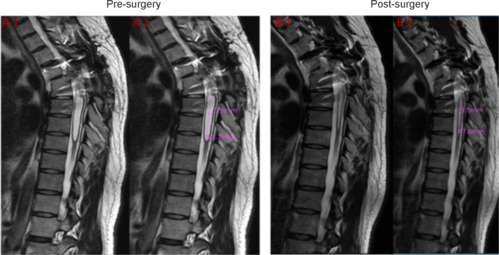
Magnetic resonance imaging before (A) and after surgery (B) in a patient in the treatment group.
(A1, A2) The preoperative MRI image of the subject 01 showing the dilation of the central canal of the spinal cord below the level of injury (A1) and the measurements of the dilation (A2). (B1, B2) The postoperative MRI of the subject showing considerable improvement in the central canal dilation (B1) and the measurements of the dilation (B2).
To evaluate whether inflammatory responses were still present over time, we examined histopathological spinal cord changes in an SCI patient who underwent surgical detethering 9 months after injury. This patient was from a different patient cohort not reported in this study. Paraformaldehye-fixed paraffin-embedded spinal cord tissue samples were shipped to Shanghai Jiao Tong University School of Medicine, where sections (4 μm) were stained with mouse anti-human CD68 (KP1, Dako, Glostrup, Denmark). Results indicated that the tissue contained KP1-positive (a marker for macrophages) activated microglia and macrophages (Figure 11). Currently, there is no effective medication to ensure the efficient and proper synchronization of the microglia-to-macrophage transition that occurs in the injured spinal cord. to a more favorable phenotype, inflammatory material was occasionally observed and, when examined microscopically contained debris-laden macrophages.
Figure 11.
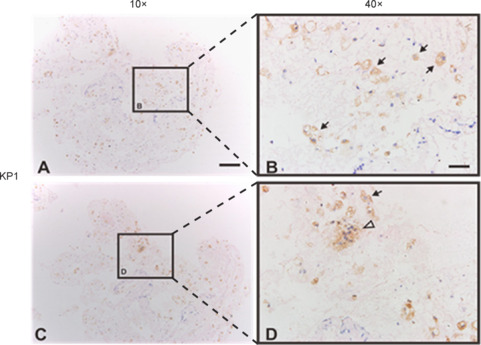
Histopathological changes in the spinal cord of a patient who underwent surgical detethering 9 months after injury.
Low (A, C) and high (B, D) magnification images. B and D represent the boxes in A and C, respectively. KP1-positive activated microglia and macrophages are visualized. Some cells exhibit amoeboid morphology. Cytoplasm filled with phagocytic vesicles is indicated by solid arrows. Activated microglia/macrophages clumped together are indicated by hollow arrows. Scale bars: 200 μm in A, C; 50 μm in B, D.
Based on our clinical experience, chronic SCI progresses steadily over years. Indeed, on MRI, we observed scarring, arachnoid cyst formation, myelomalacia, and other pathologic changes (Figure 12).
Figure 12.
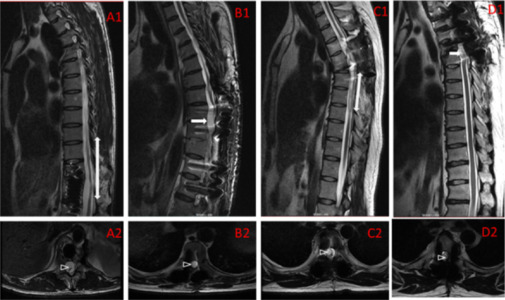
Representative MRI in a patient with chronic spinal cord injury.
Pathological changes included myeloatrophy (A1, A2), interruption of spinal cord continuity (B1, B2), syringomyelia (C1, C2), and cystic degeneration (D1, D2).
MRI assessment is necessary to decide whether surgical intervention in a patient with CSCI is warranted. Table 4 summarizes the findings which favor intervention. Detethering is not recommended in patients with chronic myeloatrophy and interrupted spinal cord continuity because these are not reversible.
Table 4.
Magnetic resonance imaging findings and recommendations
| MRI performance | Recommendation for surgery |
|---|---|
| a) Extensive adhesion of the spinal arachnoid and blockage of CSF flow | √ |
| b) Formation of arachnoid cysts, which compress the spinal cord | √ |
| c) Syringomyelia | √ |
| d) Foreign body compression(eg. bone) | √ |
| e) Cystic degeneration of the spinal cord | Optional |
| f) Interruption of spinal cord continuity | × |
| g) Myeloatrophy | × |
CSF: Cerebrospinal fluid; MRI: magnetic resonance imaging; √: surgery is recommended; ×: surgery is not recommended.
Pathological changes in the chronically injured spinal cord and how such changes can be reversed after surgical detethering are illustrated schematically in Figure 13. Fibrotic connective tissue invading the subarachnoid space can tether the spinal cord (Figure 13A). Tethering and circumferential scarring can completely block CSF flow, create CSF pressure gradients, and reduce spinal cord blood flow (Figure 13B-I). In addition, compressive subarachnoid cysts may form (Figure 13B-II). In some cases, posttraumatic syringomyelia develops and expands over several spinal cord segments (Figure 13B-III). Detethering the spinal cord and emptying fluid-filled cysts in and around the spinal cord can restore CSF circulation and possibly improve blood flow to the spinal cord (Figure 13C).
Figure 13.
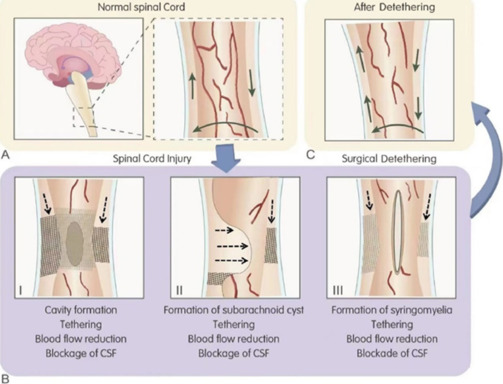
Chronic perilesional spinal cord injury patterns and effects of surgical detethering.
(A) Normal cerebrospinal fluid (CSF) flow (solid arrow). (B-I) Cavity formation in the spinal cord (oval), external tethering (grid pattern), and blockage of CSF flow (broken arrow). (B-II) Tethering (grid pattern) and blockage of CSF flow (broken arrow). (B-III) Syringomyelia formation (oval), tethering (grid pattern), blockage of CSF flow (broken arrow). (C) Improvement of CSF flow (solid arrow).
Discussion
Despite the need for treatment strategies in patients with CSCI, few studies have focused on surgical interventions in the chronic phase of injury (≥ 12 months after injury) and most are retrospective case series (Falci et al., 1999, 2009; Lee et al., 2001; Gross et al., 2013; Stenimahitis et al., 2022); one systematic review has also been reported (Moinuddin et al., 2022). We performed a randomized controlled trial to determine the efficacy of surgical intervention and weight-bearing ambulation training in the chronic phase of injury. In general, the surgical approach in patients with CSCI whose condition is stable is conservative. However, progressive pathological changes that occur in the chronic phase may reduce the capacity for functional recovery. Some of these changes are amenable to surgical treatment.
Liu et al. (2021) examined 320 SCI patients with AIS grade A cervical, thoracic, and lumbar spinal cord injuries who underwent acute (7 days) or subacute (8–30 days) intradural surgical intervention combined with weight-bearing walking training and reported benefits after both conventional bony decompression and intradural lesional decompression and early spinal cord detethering. On the basis of their study, we hypothesized that the combination of weight-bearing physical therapy and surgical detethering would improve neurological outcomes in patients with CSCI.
Our results indicate that surgical detethering of arachnoid adhesions and limited intramedullary decompression of the spinal cord in the chronic phase of injury is safe. No subject lost neurological function after surgery and no major adverse events occurred. Moreover, patients who underwent surgery participated in locomotor therapy and responded. AIS grade improved in two-thirds of subjects in the treatment group at 12 months, compared with only 1 of 15 control group subjects. This was a significant improvement over the expected natural history and over the recovery reported after acute injury in other studies (Waters et al., 1992; Zariffa et al., 2011; Aimetti et al., 2019). Mean motor and sensory score improvements were less notable than AIS grade improvement and improvements in urination and defecation. The SCIM score improvement was above the minimal clinically important difference and the Happiness Index suggested additional benefits.
Justification for spinal cord detethering in CSCI
SCI elicits an inflammatory response that comprises mainly microglia and peripheral blood-derived macrophages (Alexander and Popovich, 2009; David et al., 2012). These cells, which remain in the spinal cord for a long period of time after injury (Fleming et al., 2006; David et al., 2012), may contribute directly or indirectly to tissue damage and functional loss (Francos-Quijorna et al., 2016).
Spinal arachnoid adhesions involve adhesions of the spinal cord to the dura, nerve roots, and arachnoid membranes and may cause complete or incomplete subarachnoid space atresia, obstruction of CSF circulation, and disappearance of spinal cord pulsation. Currently, there is no effective drug or conservative treatment. Surgical dissection of the adhesions can release the spinal cord and restore circulation of CSF and spinal cord pulsatility. Likewise, for syringomyelia, detethering can decompress the syrinx, restore CSF circulation and reduce the incidence of syrinx reformation. Syringosubarachnoid shunting with a small T-tube can also be used for syrinx decompression.
A classification of changes in the chronically injured spinal cord in the era prior to routine surgical decompression of SCI was first proposed in 1996 (Wang et al., 1996). Compared with that study, our study showed less severe longitudinal atrophy. On the basis of the findings reported in the present study as well as our previous experience, we suggest a new perilesional classification of the main pathologies associated with chronic SCI based on the surgical observations (Figure 14), noting that some subjects had combinations of these parameters.
Figure 14.
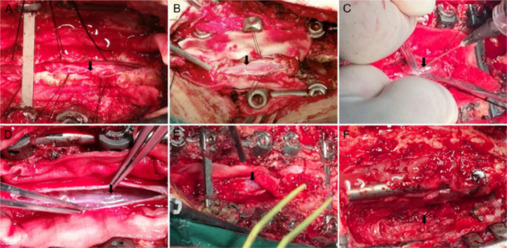
Intraoperative conditions encountered in chronic spinal cord injury (perilesional classification system).
(A) I-Adhesion. Extensive tethering adhesions of the spinal cord, spinal dura mater, or arachnoid (black arrow). The subarachnoid space is completely or incompletely occluded. Cerebrospinal (CSF) flow is blocked and pulsation of the spinal cord is absent. (B) V-Myeloatrophy. The spinal cord appears pale and atrophied (black arrow); the surface lacks a visible blood supply. (C) III-Arachnoid cysts. Cystic adhesion leads to obstruction of CSF circulation, abnormal accumulation of CSF in the subarachnoid space, and formation of cysts (black arrow) that may compress the spinal cord. (D) II-Cystic degeneration. Cystic degeneration (black arrow) of the spinal cord adjacent to the lesion may contain necrotic material, including liquified blood cells. (E) IV-Syringomyelia. Adhesions lead to CSF flow obstruction and diversion of CSF into the spinal cord parenchyma, which expands the central canal (black arrow). (F) VI-Residual compression. Bone fragments or other adjacent structures compress or distort the spinal cord. (F) VII-Spinal cord transection type. Complete rupture of the spinal cord parenchyma (black arrow) is found. Pathologic changes observed during the surgery were summarized into seven classifications. I–VII represent the classifications.
Our approach to surgery and rehabilitation in this study was consistent with the approach described in our previous study of the acute and subacute phase of injury (Liu et al., 2021). A comparison of intraoperative conditions between acute and chronic SCI phases is shown in Table 5.
Table 5.
Comparison of intraoperative conditions between chronic and acute spinal cord injury (< 30 days after injury)
| Intraoperative conditions in 15 chronic subjects | Acute SCIs |
|---|---|
| a) Adhesion of the spinal arachnoid | √ |
| b) Blockage of CSF flow | √ |
| c) Absent pulsation of the spinal cord | √ |
| d) Decreased venous plexus at spinal cord surface, and the spinal cord appeared pale | × |
| e) Arachnoid cysts | × |
| f) Interruption of spinal cord continuity | √ |
| g) Cystic degeneration of the spinal cord | √ |
| h) Syringomyelia | × |
| i) Bone compression | √ |
CSF: Cerebrospinal fluid; SCI: spinal cord injury; √: similar conditions observed in acute SCI; ×: conditions not observed in acute SCI.
Role of surgical intervention in CSCI
Surgical decompression and detethering were associated with improvement in AIS grade and faster improvement of bowel and bladder function. AIS improvement was observed within 1 month in the subjects treated with surgery and KLTP rehabilitation.
We suggest that the chronic phase of SCI is affected by myeloradiculopathy with progressive tethering and cystic compression that insidiously impedes the potential for neurological recovery. Prior reports have indicated that detethering surgery facilitates motor recovery and pain reduction (Gross et al., 2013). It also reduces mechanical stresses on the spinal cord that can compromise neurological function (Agarwal et al., 2017; Tewari et al., 2018). Surgical decompression improves spinal cord blood flow, which supports neurological recovery (Lee et al., 2002). CSF flow restoration improves spinal cord metabolism (Matsumae et al., 2016). The surgical approach in our study was applicable only to morphological abnormalities encountered at the injury site and did not involve neural regeneration treatment.
Addressing morphological abnormalities of the spinal cord by releasing arachnoid adhesions, decompressing arachnoid cysts, relieving spinal cord compression, restoring CSF circulation, and addressing syringomyelia should be considered the foundation before proceeding with neural regeneration treatment, because the abnormalities may exert a negative impact on neural regeneration.
Common surgical complications
CSF leakage is common after spinal surgery (Singh and Wang, 2012). Although the dura was closed tightly after detethering surgery, CSF leakage still occurred in one treatment group subject.
Influence of thoracic NLI on recovery
Previous studies have reported that neurological recovery is more common after T10–T12 injury than T2–T9 injury (Harrop et al., 2011; Aimetti et al., 2019). A lower level of injury spares a greater degree of trunk function and spares descending supraspinal axons closer to the sacral motor neurons responsible for bowel and bladder function.
Locomotor recovery without significant change in ISNCSCI motor score
Over-ground locomotion requires movement synergy and continuous sensory feedback. Locomotor rehabilitation has been shown to facilitate post-SCI recovery (Harkema et al., 2012; Field-Fote et al., 2017; Magnuson and Dietrich, 2017; Sinovas-Alonso et al., 2021; Quinta, 2022) via retraining of the residual spinal stepping circuitry (pattern generator) (Magnuson and Dietrich, 2017; Moreno-López and Hollis, 2021). Bélanger et al. (1996) reported that intact but isolated locomotor circuitry below a complete lower thoracic transection in cats supports near normal hindlimb stepping during treadmill training. In rodent studies of full-weight-bearing treadmill locomotion, non-functional circuits can transform into functional circuits below a complete transection (Courtine et al., 2009).
Task specificity is a significant factor to consider in improving real-world walking with locomotor training. Evidence has emerged that the emulation of actual conditions is critical (Kuerzi et al., 2010). In human subjects with complete SCI, the spinal circuitry can generate motor patterns effective for standing in response to task-specific training during optimized epidural stimulation. However, focusing on step training can lead to neural adaptations which result in impairment of the prior standing recovery (Rejc et al., 2017). KLTP is individualized to the subject and provides limb-stabilizing support that is specific to walking without technological assistance.
KLTP is unique in several ways. First, over-ground training involves progressive increases in weight-bearing and challenges balance under the careful supervision of experienced therapists. Second, KLTP individually optimizes the step cycles as required by each patient’s individual function. Last, the training is intensive (3 hours per day, 5 days per week) and takes place over a long period of time. During KLTP training, various weight-bearing devices are used, combined with foot drop prevention and stabilization of the knee. The rapid early improvement of locomotion during KLTP may be due to the individualized correction of deficits and relearning of standing and walking. Early improvements are facilitated by the correction of foot drop, knee fixation, and upper-body weight support. During KLTP, therapists have noticed that muscle strength appears to improve in the upper limbs and thoracoabdominal core and that weight-bearing and ambulation quality also improve. This program of locomotor-supported training is effective despite limited changes in motor and sensory scores, whereas recovery of KLTP and WISCI scores was significant and similar. We propose that motor and sensory scores obtained while supine may underestimate the potential for useful locomotor synergies during weight-bearing.
Limitations
Our study has several limitations. The sample size was relatively small, potentially underpowering the detection of some effects. In contrast to most previous studies of detethering in the chronic phase of SCI, clinical neurological deterioration before surgery was not required for enrollment. Such deterioration is not easily detected in patients with AIS grade A SCI. Likewise, detection of adverse neurological change after thoracic level surgery is limited to sensory level assessment and assessment of less objective variables such as changes in pain and spasticity. Although the treatment program and associated clinical evaluations were conducted over 12 months, most changes occurred within 2 to 3 months. Perhaps a shorter program would be effective. Intensive rehabilitation may be associated with positive systemic effects such as improvements in metabolism and social interactions, but these were not studied.
Conclusion
This study suggests that surgery and rehabilitation have a role in treatment of SCI in the chronic phase, with the premise that progressive pathological changes in the spinal cord after injury limit functional recovery, especially bladder and bowel recovery. Therefore, restricting surgical intervention to only CSCI patients with progressive neurological loss of function may be overly conservative when significant MRI abnormalities are present.
Our study demonstrates that long-term weight-bearing locomotor training combined with intradural/intramedullary surgical detethering and decompression in patients with complete SCI is associated with improvement in AIS grade, improved bowel and bladder function, reduced spasticity, recovery of locomotor function, and increased independence and well-being. CSCI patients with a completely interrupted spinal cord or myeloatrophy on MRI are not recommended for surgical detethering, because these pathological abnormalities cannot be improved.
The intraoperative findings and related surgical procedures described in this study are important for guiding clinical practice and future CSCI research. Replication of our findings in larger-scale studies is needed, as is an evaluation of whether the benefits of surgical intervention in combination with intensive rehabilitation are sustained over the long-term.
Acknowledgments:
All physicians and clinical staff at Kunming Tongren Hospital involved in this trial are acknowledged for their dedication to the subject treatments and daily care. We also thank the members of the International Review Committee for their helpful program reviews, valuable suggestions, and critique. Thanks to Mrs Suzanne Poon, chair of the Hong Kong Spinal Cord Injury Fund (HKSCIF) and all the personnel of the Fund for their tremendous support of the trial. Thanks to Ms. Xiaoqing Feng (Kunming Tongren Hospital) for translation. This article is in memory of Xiao-Ming Xu, excellent professor at Indiana University School of Medicine and editor-in-chief of the Neural Regeneration Research journal, who dedicated most of his life to spinal cord injury research.
Funding Statement
Funding: This study was supported by Hong Kong Spinal Cord Injury Fund (HKSCIF), China (to HZ).
Footnotes
Conflicts of interest: The authors declare that they have no conflicts of interest.
Editor note: The corresponding author KFS is the Editor-in-Chief of Neural Regeneration Research. KFS did not participate in the peer review of decision-making of the review.
SD, WW, and WY are Editorial Board members of Neural Regeneration Research. They were blinded from reviewing or making decisions on the manuscript. The article was subject to the journal’s standard procedures, with peer-review handled independently of these Editorial Board and Editorial Office members and their research groups.
Data availability statement: No additional data are available.
C-Editor: Zhao M; S-Editor: Li CH; L-Editors: Li CH, Song LP; T-Editor: Jia Y
References
- Agarwal N, Hansberry DR, Goldstein IM. Tethered cord as a complication of chronic cerebral spinal fluid diversion. Int J Spine Surg. 2017;11:26. doi: 10.14444/4026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aimetti AA, Kirshblum S, Curt A, Mobley J, Grossman RG, Guest JD. Natural history of neurological improvement following complete (AIS A) thoracic spinal cord injury across three registries to guide acute clinical trial design and interpretation. Spinal Cord. 2019;57:753–762. doi: 10.1038/s41393-019-0299-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akpinar P, Atici A, Ozkan FU, Aktas I, Kulcu DG, Sarı A, Durmus B. Reliability of the Modified Ashworth Scale and Modified Tardieu Scale in patients with spinal cord injuries. Spinal Cord. 2017;55:944–949. doi: 10.1038/sc.2017.48. [DOI] [PubMed] [Google Scholar]
- Alexander JK, Popovich PG. Neuroinflammation in spinal cord injury: Therapeutic targets for neuroprotection and regeneration. Prog Brain Res. 2009;175:125–137. doi: 10.1016/S0079-6123(09)17508-8. [DOI] [PubMed] [Google Scholar]
- Anderson KD, Acuff ME, Arp BG, Backus D, Chun S, Fisher K, Fjerstad JE, Graves DE, Greenwald K, Groah SL, Harkema SJ, Horton JA, 3rd, Huang MN, Jennings M, Kelley KS, Kessler SM, Kirshblum S, Koltenuk S, Linke M, Ljungberg I, et al. United States (US) multi-center study to assess the validity and reliability of the Spinal Cord Independence Measure (SCIM III) Spinal Cord. 2011;49:880–885. doi: 10.1038/sc.2011.20. [DOI] [PubMed] [Google Scholar]
- Badhiwala JH, Wilson JR, Fehlings MG. Global burden of traumatic brain and spinal cord injury. Lancet Neurol. 2019;18:24–25. doi: 10.1016/S1474-4422(18)30444-7. [DOI] [PubMed] [Google Scholar]
- Bélanger M, Drew T, Provencher J, Rossignol S. A comparison of treadmill locomotion in adult cats before and after spinal transection. J Neurophysiol. 1996;76:471–491. doi: 10.1152/jn.1996.76.1.471. [DOI] [PubMed] [Google Scholar]
- Catz A, Itzkovich M, Agranov E, Ring H, Tamir A. SCIM--spinal cord independence measure: a new disability scale for patients with spinal cord lesions. Spinal Cord. 1997;35:850–856. doi: 10.1038/sj.sc.3100504. [DOI] [PubMed] [Google Scholar]
- Catz A, Itzkovich M, Tesio L, Biering-Sorensen F, Weeks C, Laramee MT, Craven BC, Tonack M, Hitzig SL, Glaser E, Zeilig G, Aito S, Scivoletto G, Mecci M, Chadwick RJ, El Masry WS, Osman A, Glass CA, Silva P, Soni BM, et al. A multicenter international study on the Spinal Cord Independence Measure, version III: Rasch psychometric validation. Spinal Cord. 2007;45:275–291. doi: 10.1038/sj.sc.3101960. [DOI] [PubMed] [Google Scholar]
- Courtine G, Gerasimenko Y, van den Brand R, Yew A, Musienko P, Zhong H, Song B, Ao Y, Ichiyama RM, Lavrov I, Roy RR, Sofroniew MV, Edgerton VR. Transformation of nonfunctional spinal circuits into functional states after the loss of brain input. Nat Neurosci. 2009;12:1333–1342. doi: 10.1038/nn.2401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- David S, Lopez-Vales R, Wee Yong V. Harmful and beneficial effects of inflammation after spinal cord injury: Potential therapeutic implications. Handb Clin Neurol. 2012;109:485–502. doi: 10.1016/B978-0-444-52137-8.00030-9. [DOI] [PubMed] [Google Scholar]
- Dittuno PL, Ditunno JF., Jr Walking index for spinal cord injury (WISCI II): scale revision. Spinal Cord. 2001;39:654–656. doi: 10.1038/sj.sc.3101223. [DOI] [PubMed] [Google Scholar]
- Ditunno JF, Jr, Formal CS. Chronic spinal cord injury. N Engl J Med. 1994;330:550–556. doi: 10.1056/NEJM199402243300808. [DOI] [PubMed] [Google Scholar]
- Ditunno JF, Jr, Ditunno PL, Scivoletto G, Patrick M, Dijkers M, Barbeau H, Burns AS, Marino RJ, Schmidt-Read M. The Walking Index for Spinal Cord Injury (WISCI/WISCI II): nature, metric properties, use and misuse. Spinal Cord. 2013;51:346–355. doi: 10.1038/sc.2013.9. [DOI] [PubMed] [Google Scholar]
- Falci SP, Lammertse DP, Best L, Starnes CA, Prenger EC, Stavros AT, Mellick D. Surgical treatment of posttraumatic cystic and tethered spinal cords. J Spinal Cord Med. 1999;22:173–181. doi: 10.1080/10790268.1999.11719567. [DOI] [PubMed] [Google Scholar]
- Falci SP, Indeck C, Lammertse DP. Posttraumatic spinal cord tethering and syringomyelia: surgical treatment and long-term outcome. J Neurosurg Spine. 2009;11:445–460. doi: 10.3171/2009.4.SPINE09333. [DOI] [PubMed] [Google Scholar]
- Field-Fote EC, Yang JF, Basso DM, Gorassini MA. Supraspinal control predicts locomotor function and forecasts responsiveness to training after spinal cord injury. J Neurotrauma. 2017;34:1813–1825. doi: 10.1089/neu.2016.4565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleming JC, Norenberg MD, Ramsay DA, Dekaban GA, Marcillo AE, Saenz AD, Pasquale-Styles M, Dietrich WD, Weaver LC. The cellular inflammatory response in human spinal cords after injury. Brain. 2006;129:3249–3269. doi: 10.1093/brain/awl296. [DOI] [PubMed] [Google Scholar]
- Francos-Quijorna I, Amo-Aparicio J, Martinez-Muriana A, López-Vales R. IL-4 drives microglia and macrophages toward a phenotype conducive for tissue repair and functional recovery after spinal cord injury. Glia. 2016;64:2079–2092. doi: 10.1002/glia.23041. [DOI] [PubMed] [Google Scholar]
- Gross R, Hamel O, Robert R, Perrouin-Verbe B. Perilesional myeloradiculopathy with tethered cord in post-traumatic spinal cord injury. Spinal Cord. 2013;51:369–374. doi: 10.1038/sc.2012.154. [DOI] [PubMed] [Google Scholar]
- Haas BM, Bergström E, Jamous A, Bennie A. The inter rater reliability of the original and of the modified Ashworth scale for the assessment of spasticity in patients with spinal cord injury. Spinal Cord. 1996;34:560–564. doi: 10.1038/sc.1996.100. [DOI] [PubMed] [Google Scholar]
- Harkema S, Behrman A, Barbeau H. Evidence-based therapy for recovery of function after spinal cord injury. Handb Clin Neurol. 2012;109:259–274. doi: 10.1016/B978-0-444-52137-8.00016-4. [DOI] [PubMed] [Google Scholar]
- Harrop JS, Naroji S, Maltenfort MG, Ratliff JK, Tjoumakaris SI, Frank B, Anderson DG, Albert T, Vaccaro AR. Neurologic improvement after thoracic, thoracolumbar, and lumbar spinal cord (conus medullaris) injuries. Spine (Phila Pa 1976) 2011;36:21–25. doi: 10.1097/BRS.0b013e3181fd6b36. [DOI] [PubMed] [Google Scholar]
- Holmes D. Repairing the neural highway. Nature. 2017;552:S50–51. doi: 10.1038/d41586-017-07551-8. [DOI] [PubMed] [Google Scholar]
- Kuerzi J, Brown EH, Shum-Siu A, Siu A, Burke D, Morehouse J, Smith RR, Magnuson DS. Task-specificity vs. ceiling effect: step-training in shallow water after spinal cord injury. Exp Neurol. 2010;224:178–187. doi: 10.1016/j.expneurol.2010.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JH, Chung CK, Kim HJ. Decompression of the spinal subarachnoid space as a solution for syringomyelia without Chiari malformation. Spinal Cord. 2002;40:501–506. doi: 10.1038/sj.sc.3101322. [DOI] [PubMed] [Google Scholar]
- Lee TT, Alameda GJ, Camilo E, Green BA. Surgical treatment of post-traumatic myelopathy associated with syringomyelia. Spine (Phila Pa 1976) 2001;26:S119–127. doi: 10.1097/00007632-200112151-00020. [DOI] [PubMed] [Google Scholar]
- Li J, Liu X, Wang J, Wang F, Zhu Z, Tang T, Wang J, Zhou Z, Gao M, Liu S. Identification of immunodiagnostic blood biomarkers associated with spinal cord injury severity. Front Immunol. 2023;14:1101564. doi: 10.3389/fimmu.2023.1101564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Walker CL, Zhang YP, Shields CB, Xu XM. Surgical decompression in acute spinal cord injury: A review of clinical evidence, animal model studies, and potential future directions of investigation. Front Biol (Beijing) 2014;9:127–136. doi: 10.1007/s11515-014-1297-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu NK, Zhang YP, Titsworth WL, Jiang X, Han S, Lu PH, Shields CB, Xu XM. A novel role of phospholipase A2 in mediating spinal cord secondary injury. Ann Neurol. 2006;59:606–619. doi: 10.1002/ana.20798. [DOI] [PubMed] [Google Scholar]
- Liu NK, Zhang YP, Han S, Pei J, Xu LY, Lu PH, Shields CB, Xu XM. Annexin A1 reduces inflammatory reaction and tissue damage through inhibition of phospholipase A2 activation in adult rats following spinal cord injury. J Neuropathol Exp Neurol. 2007;66:932–943. doi: 10.1097/nen.0b013e3181567d59. [DOI] [PubMed] [Google Scholar]
- Liu Y, Xie JX, Niu F, Xu Z, Tan P, Shen C, Gao H, Liu S, Ma Z, So KF, Wu W, Chen C, Gao S, Xu XM, Zhu H. Surgical intervention combined with weight-bearing walking training improves neurological recoveries in 320 patients with clinically complete spinal cord injury: a prospective self-controlled study. Neural Regen Res. 2021;16:820–829. doi: 10.4103/1673-5374.297080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magnuson DSK, Dietrich WD. Introduction to the special issue on locomotor rehabilitation after spinal cord injury. J Neurotrauma. 2017;34:1711–1712. doi: 10.1089/neu.2017.5126. [DOI] [PubMed] [Google Scholar]
- Malekzadeh H, Golpayegani M, Ghodsi Z, Sadeghi-Naini M, Asgardoon M, Baigi V, Vaccaro AR, Rahimi-Movaghar V. Direct cost of illness for spinal cord injury: a systematic review. Global Spine J. 2022;12:1267–1281. doi: 10.1177/21925682211031190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin JH. Neuroplasticity of spinal cord injury and repair. Handb Clin Neurol. 2022;184:317–330. doi: 10.1016/B978-0-12-819410-2.00017-5. [DOI] [PubMed] [Google Scholar]
- Matsumae M, Sato O, Hirayama A, Hayashi N, Takizawa K, Atsumi H, Sorimachi T. Research into the physiology of cerebrospinal fluid reaches a new horizon: intimate exchange between cerebrospinal fluid and interstitial fluid may contribute to maintenance of homeostasis in the central nervous system. Neurol Med Chir (Tokyo) 2016;56:416–441. doi: 10.2176/nmc.ra.2016-0020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moinuddin FM, Yolcu YU, Wahood W, Zreik J, Goncalves S, Windebank AJ, Qu W, Bydon M. Time-to-enrollment in clinical trials investigating neurological recovery in chronic spinal cord injury: observations from a systematic review and ClinicalTrials.gov database. Neural Regen Res. 2022;17:953–958. doi: 10.4103/1673-5374.324826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreno-López Y, Hollis ER. Sensory circuit remodeling and movement recovery after spinal cord injury. Front Neurosci. 2021;15:787690. doi: 10.3389/fnins.2021.787690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- No authors listed Spinal Cord Injury (SCI) 2016 facts and figures at a glance. J Spinal Cord Med. 2016;39:493–494. doi: 10.1080/10790268.2016.1210925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Donovan KJ. Intrinsic axonal growth and the drive for regeneration. Front Neurosci. 2016;10:486. doi: 10.3389/fnins.2016.00486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quinta HR. Locomotor recovery after spinal cord injury: intimate dependence between axonal regeneration and re-connection. Neural Regen Res. 2022;17:553–554. doi: 10.4103/1673-5374.320977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rejc E, Angeli CA, Bryant N, Harkema SJ. Effects of stand and step training with epidural stimulation on motor function for standing in chronic complete paraplegics. J Neurotrauma. 2017;34:1787–1802. doi: 10.1089/neu.2016.4516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren Y, Young W. Managing inflammation after spinal cord injury through manipulation of macrophage function. Neural Plast. 2013;2013:945034. doi: 10.1155/2013/945034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rupp R, Biering-Sørensen F, Burns SP, Graves DE, Guest J, Jones L, Read MS, Rodriguez GM, Schuld C, Tansey-Md KE, Walden K, Kirshblum S. International standards for neurological classification of spinal cord injury: revised 2019. Top Spinal Cord Inj Rehabil. 2021;27:1–22. doi: 10.46292/sci2702-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulz KF, Altman DG, Moher D, Group C. CONSORT 2010 Statement: updated guidelines for reporting parallel group randomised trials. Trials. 2010;11:32. doi: 10.4103/0976-500X.72352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi F, Zhu H, Yang S, Liu Y, Feng Y, Shi J, Xu D, Wu W, You S, Ma Z, Zou J, Lu P, Xu XM. Glial response and myelin clearance in areas of wallerian degeneration after spinal cord hemisection in the monkey Macaca fascicularis. J Neurotrauma. 2009;26:2083–2096. doi: 10.1089/neu.2008.0706. [DOI] [PubMed] [Google Scholar]
- Singh H, Wang MY. Cerebrospinal fluid leakage and management: a literature review. ASN J. 2012;24:188–193. [Google Scholar]
- Sinovas-Alonso I, Gil-Agudo Á, Cano-de-la-Cuerda R, Del-Ama AJ. Walking Ability Outcome Measures in Individuals with Spinal Cord Injury: A Systematic Review. Int J Environ Res Public Health. 2021;18:9517. doi: 10.3390/ijerph18189517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stenimahitis V, Fletcher-Sandersjöö A, Tatter C, Elmi-Terander A, Edström E. Long-term outcome following surgical treatment of posttraumatic tethered cord syndrome: a retrospective population-based cohort study. Spinal Cord. 2022;60:516–521. doi: 10.1038/s41393-022-00752-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tewari VK, Somvanshi R, Trivedi RB, Hussain M, Das Gupta HK, Dubey RS. Pure tethered cervical cord and review of literature. Asian J Neurosurg. 2018;13:72–74. doi: 10.4103/1793-5482.224834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker CL, Walker MJ, Liu NK, Risberg EC, Gao X, Chen J, Xu XM. Systemic bisperoxovanadium activates Akt/mTOR, reduces autophagy, and enhances recovery following cervical spinal cord injury. PLoS One. 2012;7:e30012. doi: 10.1371/journal.pone.0030012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scivoletto G, Tamburella F, Laurenza L, Molinari M. The spinal cord independence measure: how much change is clinically significant for spinal cord injury subjects. Disabil Rehabil. 2013;35:1808–1813. doi: 10.3109/09638288.2012.756942. [DOI] [PubMed] [Google Scholar]
- Wang D, Bodley R, Sett P, Gardner B, Frankel H. A clinical magnetic resonance imaging study of the traumatised spinal cord more than 20 years following injury. Paraplegia. 1996;34:65–81. doi: 10.1038/sc.1996.13. [DOI] [PubMed] [Google Scholar]
- Waters RL, Yakura JS, Adkins RH, Sie I. Recovery following complete paraplegia. Arch Phys Med Rehabil. 1992;73:784–789. [PubMed] [Google Scholar]
- Wu W, Wu W, Zou J, Shi F, Yang S, Liu Y, Lu P, Ma Z, Zhu H, Xu XM. Axonal and glial responses to a mid-thoracic spinal cord hemisection in the Macaca fascicularis monkey. J Neurotrauma. 2013;30:826–839. doi: 10.1089/neu.2012.2681. [DOI] [PubMed] [Google Scholar]
- Young W. Spinal cord regeneration. Cell Transplant. 2014;23:573–611. doi: 10.3727/096368914X678427. [DOI] [PubMed] [Google Scholar]
- Zariffa J, Kramer JL, Fawcett JW, Lammertse DP, Blight AR, Guest J, Jones L, Burns S, Schubert M, Bolliger M, Curt A, Steeves JD. Characterization of neurological recovery following traumatic sensorimotor complete thoracic spinal cord injury. Spinal Cord. 2011;49:463–471. doi: 10.1038/sc.2010.140. [DOI] [PubMed] [Google Scholar]
- Zhu H, Poon W, Liu Y, Leung GK, Wong Y, Feng Y, Ng SCP, Tsang KS, Sun DTF, Yeung DK, Shen C, Niu F, Xu Z, Tan P, Tang S, Gao H, Cha Y, So KF, Fleischaker R, Sun D, et al. Phase I-II clinical trial assessing safety and efficacy of umbilical cord blood mononuclear cell transplant therapy of chronic complete spinal cord injury. Cell Transplant. 2016;25:1925–1943. doi: 10.3727/096368916X691411. [DOI] [PubMed] [Google Scholar]


