Abstract
Purpose:
The study was undertaken to look into the clinicodemographic profile, management, and clinical outcomes of advanced retinoblastoma at a tertiary care center.
Methods:
A prospective cohort study was conducted from Jan 2019 to Dec 2022. Forty-two patients of intraocular advanced retinoblastoma were assessed. The treatment protocol was formulated based on size, extension of tumor, and laterality. Primary outcome measure was response to the treatment in terms of regression of tumor and seeds and no evidence of recurrence after 12 month in enucleated eyes. Secondary outcome measures were complications like implant exposure, metastasis, and death associated with each treatment modality.
Results:
The mean age of the study group was 13 months. The most common presentation was leukocoria with diminished vision. Most of the patients had group E retinoblastoma (n = 40, 95%) as per the International Classification of Retinoblastoma. In 12 patients with group E retinoblastoma, primary enucleation was performed and in six patients, secondary enucleation was done, in which initially, globe salvage treatment was tried. In 30 patients, globe salvage treatment was attempted and we could manage to save 23 eyes. The most common treatment modality was intra-arterial chemotherapy using a triple-drug regimen. One patient developed intracranial spread and died due to systemic metastasis during the follow-up period.
Conclusion:
The current study showed that globe salvage is possible in advanced retinoblastoma if appropriate therapy is instituted depending upon the extent of the tumor and availability of latest treatment modalities. Intra-arterial chemotherapy using triple drugs can be offered as a first-line therapy in advanced unilateral retinoblastoma as it has been found to be very effective in the present study.
Keywords: Enucleation, eye, intra-arterial chemotherapy, retinoblastoma, salvage
Retinoblastoma is the most common primary intraocular malignant tumor in children, with a global incidence of approximately 8000-9000 annually.[1,2] Incidence in India is about 1 in 15,000–18,000 live births.[3] The management of retinoblastoma is intricate and challenging, which often requires a multidisciplinary approach. The main aim of treatment in retinoblastoma is to save life first, then globe salvage, and, if possible, preservation of vision.[4,5] In the Indian population, the overall mortality is 24% and the cumulative 5-year survival probability is 65%.[6]
Eye salvage is possible in advanced retinoblastoma in the present era due to advances in the management of retinoblastoma.[7,8,9] This has become feasible because of the inclusion of newer treatment modalities like intra-arterial chemotherapy, intravitreal chemotherapy, and plaque brachytherapy using iodine and ruthenium plaque.[10,11] This has given a ray of hope of tackling this malignant tumor effectively and has reduced morbidity and mortality associated with this disease. But there is disparity in this rate in developed and developing countries, as in developing countries, patients have poor access to advanced treatment facility due to its nonavailability at all places and lack of financial support.[12,13] Eye salvage in advanced retinoblastoma is also marred by many controversies. First, there is a risk of metastatic spread as complete regression of the tumor is possible with multiple sessions of chemotherapy and during this time, there is a risk of hematogenous and intracranial spread. Second, this treatment is not free of side effects, which is not justified if the visual prognosis is poor in the affected eye.[14,15] There are very few studies which have reported about the outcomes in advanced retinoblastoma using these newer treatment methods or techniques because of the complexities associated with treatment of advanced retinoblastoma. Hence, this study was undertaken at our center to delve into the clinicodemographic profile, management, and treatment outcomes in advanced retinoblastoma among the Indian population.
Methods
A prospective study was conducted from Jan 2019 to Dec 2022 at a tertiary care center in the northern part of India. Totally, 42 patients diagnosed with intraocular retinoblastoma were seen during this period. As per the International Classification of Retinoblastoma (ICRB), advanced retinoblastoma is defined as Group D or E. Group D retinoblastoma is retinoblastoma with subretinal seeds >3 mm from the tumor or vitreous seeds >3 mm from the tumor or both subretinal seeds and vitreous seeds >3 mm from retinoblastoma. Group E retinoblastoma is extensive retinoblastoma occupying >50% of the globe, with presence of neovascular glaucoma, opaque media from hemorrhage in the anterior chamber, vitreous or subretinal space, and invasion of postlaminar optic nerve, choroid (>2 mm), sclera, orbit, and anterior chamber. The study was approved by the institutional review board and adhered to the tenets of the declaration of Helsinki. Informed consent was taken from the next of kin in all cases. All cases were diagnosed on the basis of clinical presentation, complete ocular examination, and imaging, mainly B scan ultrasonography and magnetic resonance imaging (MRI) orbit. Retinal images were captured with RetCam in all cases. Patients with advanced retinoblastoma who met the ICRB criteria and who were compliant with follow-up were included in the study group. Patients with group A, B, C retinoblastoma and who were treated somewhere else were excluded from the study. Patients with group E retinoblastoma with neovascular glaucoma (NVG), anterior chamber involvement, and phthisis bulbi were not considered for primary intra-arterial chemotherapy. All patients were followed up for a minimum of 1 year at our center. Data like age of the patient, gender, residential address, duration of symptoms, family history of retinoblastoma, history of prior systemic condition, details of previous treatment history, and presenting complaints were recorded. All ocular examinations were done under general anesthesia. Cerebrospinal fluid aspiration and bone marrow biopsy were done in group D and group E where optic disc was not visible. Positron emission tomography scan was done in all advanced cases of retinoblastoma to rule out systemic involvement.
Treatment plan
Being a tertiary care center, the horizon of treatment armamentarium is broad at our center, which includes chemotherapy through different routes, transpupillary thermotherapy, cryotherapy, plaque brachytherapy with indigenous ruthenium plaque, external beam radiotherapy, enucleation with implant by myoconjunctival technique, and orbital exenteration. Indigenous ruthenium plaque 106 used at this center was procured from Bhabha Atomic Research Center. At our center, we formulated our own treatment protocol based on the laterality, extent of the lesion, and the results of already published studies. The main aim of treatment at our center was to salvage the globe even in advanced intraocular retinoblastoma. In bilateral cases, we administered 12 cycles of systemic chemotherapy along with focal consolidation with transpupillary thermotherapy and cryotherapy. Twenty-seven cases of advanced intraocular retinoblastoma received intra-arterial chemotherapy using triple-drug regimen drugs as a first-line therapy. Intra-arterial chemotherapy (IAC) was given every 4 weeks for three sessions in all cases. Plaque brachytherapy using indigenous ruthenium plaque was performed when the basal diameter of the tumor was less than 16 mm and thickness was less than 8 mm. Multifocal tumors associated with active diffuse vitreous and subretinal seeds should not be considered for plaque brachytherapy. Primary enucleation with implant was advised in group E patients who presented with neovascular glaucoma, phthisis bulbi, and parents who opted for this treatment modality, and secondary enucleation was recommended when the patient did not respond to three sessions of IAC in primary and recurrent tumor. We followed the orbital protocol in orbital retinoblastoma, which included six cycles of chemotherapy followed by enucleation or exenteration, then external beam radiotherapy, and another six cycles of systemic chemotherapy. Examination was repeated every 4 weeks after each cycle of chemotherapy, and patients were followed up for 1 year. At each visit, fundus images were taken with RetCam for comparison. Adjuvant focal therapy was done using 810 diode laser or cryotherapy depending upon the location of the tumor and repeated with every examination under anesthesia until tumor control was completely achieved.
Statistical methods
Statistical analysis was done by using Epi Info version 3.4.3 and entering the data in Microsoft Excel 2007. Descriptive statistics such as percentages, mean, and standard deviation were calculated.
Primary outcome measure was response to the treatment in terms of regression of tumor and seeds and no sign of recurrence after 12 months in enucleated eyes.
Secondary outcome measures were complications like implant exposure, metastasis, and death associated with each treatment technique.
Results
A total of 42 patients were seen during the study period. All patients had advanced retinoblastoma at the time of presentation and were referred from peripheral hospitals. They were residents of northern and western parts of India. Two patients out of 42 patients had bilateral retinoblastoma. The demographic and clinical profile of patients is presented in Table 1. The mean age of patients in the current study was 13 months (range 8–36 months). There were 23 females and 19 males. Right eye was involved in 26 patients and left eye in 16 patients. Leukocoria was the common presentation in all cases with diminished vision (100%) [Fig. 1a, red arrow], followed by strabismus in 10 cases (23.8%) and bulging eyes in one patient (2%). One patient (2%) presented with proptosis and redness, and one patient had a family history of retinoblastoma. Advanced intraocular retinoblastoma tumors were classified as group D (n = 2, 4.7%) or group E (n = 40, 95%) based on the ICRB criteria [Table 1]. Maximum number of patients in the current study had unilateral group E retinoblastoma [Fig. 1b].
Table 1.
Demographic profile, clinical and histopathologic features of advanced retinoblastoma
| Features No. of patients (n) | Percentage (%) | |||
|---|---|---|---|---|
| Age at the initial presentation (months) Mean (median, range) Gender Male Female Laterality Unilateral Bilateral Affected eye Right eye Left eye Both eyes Family history of retinoblastoma Common clinical presentation Leukocoria Diminished vision Squint Bulging eyes Redness Pain Endophytic lesion Orbital cellulitis Raised intraocular pressure Iris neovascularization ICRB Group D Group E Combination of group E and group B AJCC Clinical Staging, 8th ed.ition T2a T2b T3b T3c T3d T4b Combination of T1 and T2 Histological High-risk features Primary enucleation Secondary enucleation |
13 months (12, 8–36) 19 23 40 02 25 15 02 01 42 42 10 01 01 01 42 01 05 02 02 40 02 02 40 02 07 01 01 02 03 02 |
45.2 54.76 95.2 4.76 59.52 35.71 4.76 2.38 100 100 23.8 2.38 2.38 2.38 100 2.38 11.9 4.76 4.76 95.2 4.76 4.76 95.2 4.76 16.6 2.38 2.38 4.76 7.14 4.76 |
||
ICRB=International classification of retinoblastoma, AJCC=American joint committee on cancer
Figure 1.
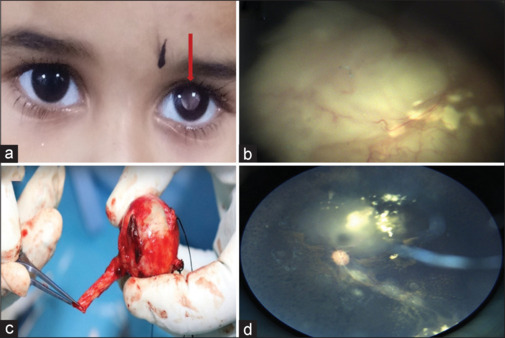
(a) Clinical photograph illustrating white reflex in the left eye (red arrow). (b) Fundus image showing group E retinoblastoma with a large endophytic tumor and diffuse subretinal fluid. (c) Clinical image showing enucleated eyeball with 15-mm optic nerve. (d) Fundus image showing regressed tumor (Type 3) following three sessions of intra-arterial chemotherapy
Details of the treatment received by patients in the current study are presented in Table 2. In unilateral group E patients, both enucleation and IAC were offered to parents, except one patient who had orbital spread at the time of presentation. Twelve patients (28%) underwent primary enucleation [Fig. 1c] since their parents opted for this therapy as it was not possible for them to visit this center for follow-up every 3 weeks, being from far-flung areas of the country. Three patients (25%) in this group were found to have high-risk features as histopathologic examination showed involvement of retrolaminar part of the optic nerve. All three patients received adjuvant six cycles of chemotherapy and did not show any signs of metastasis during follow-up. Implant extrusion was found in one case and MRI was repeated, which showed recurrence of tumor near the optic nerve. This patient received adjuvant six cycles of chemotherapy followed by external beam radiotherapy to the affected orbit. Treatment for globe salvage was attempted in 30 patients of advanced retinoblastoma. Systemic chemotherapy was given for three patients as two patients had bilateral presentation and one patient had orbital involvement. The chemotherapeutic drugs were intravenous (IV) carboplatin, vincristine, and etoposide. We could manage to salvage the globe only in one patient among bilateral retinoblastoma patients. The mean number of systemic chemotherapy cycles for complete regression of the tumor was six cycles and they were repeated every 3 weeks. Intra-arterial chemotherapy using three drugs (melphalan, topotecan, carboplatin) was administered as a first-line therapy in 27 patients as all were unilateral cases of advanced retinoblastoma. On an average, all patients in the IAC group received three cycles of IAC. Focal consolidation was done after chemoreduction with transpupillary thermotherapy and cryotherapy, depending upon the location of the tumor in five patients after three cycles of IAC. We could manage to save the globe in 22 patients who received IAC [Fig. 1d]. Failure to this therapy was considered when patients did not show any sign of regression of tumor even after three cycles of chemotherapy. Five patients in this group underwent secondary enucleation as the tumor did not respond to three sessions of IAC in three cases and due to recurrence of the tumor in two cases. Recurrence was found in five cases, which was due to activation of the vitreous or subretinal seeds in three cases [Fig. 2a] and activation of the tumor itself in two cases [Fig. 2e]. Intravitreal melphalan was administered for active vitreous seeds in one case. The dose of intravitreal melphalan was 20 µg and it was repeated every 2 weeks for 3 months, which resulted in regression of vitreous seeds [Fig. 2b]. Plaque brachytherapy using indigenous ruthenium plaque was performed in two recurrent cases due to activation of the tumor itself [Fig. 2c and d]. The basal diameter of the tumor in this case was less than 16 mm and thickness was less than 8 mm. The tumor got regressed and did not show any sign of recurrence at 6 months of follow-up [Fig. 2f]. Secondary enucleation using myoconjunctival technique was done in two recurrent cases. Treatment outcomes have been presented in Table 3.
Table 2.
Various treatment modalities used in the current study
| Treatment group (intervention) | No. of patients (n) | Percentage (%) | ||
|---|---|---|---|---|
| Primary enucleation Globe salvage treatment Systemic chemotherapy Intra-arterial chemotherapy Focal therapy TTT Cryotherapy Intravitreal chemotherapy Plaque brachytherapy Secondary enucleation |
12 30 03 27 03 02 01 02 06 |
28.57 71.42 10 64.28 10 6.66 3.33 6.6 20 |
TTT=Transpupillary Thermotherapy
Figure 2.
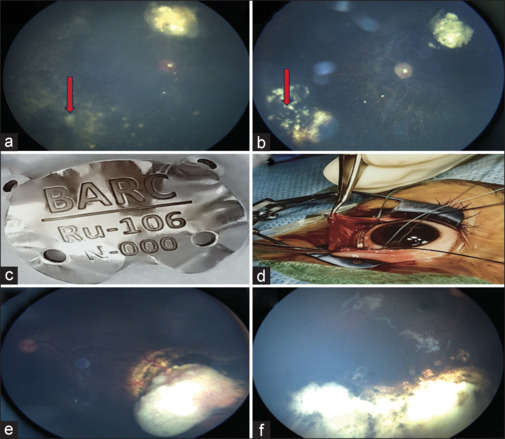
(a) Fundus image showing active vitreous seeds (red arrow). (b) Fundus image showing regressed and calcified vitreous seeds following three sessions of intravitreal melphalan. (c) Clinical photograph showing indigenous ruthenium plaque. (d) Intraoperative image showing the ruthenium plaque over the tumor. (e) Fundus photograph showing recurrence of tumor in the inferotemporal quadrant over the edge of chorioretinal scar of cryotherapy with feeder vessel at the periphery with intrinsic vascularity. (f) Fundus photograph showing completely regressed tumor after 1 month
Table 3.
Clinical outcomes in our study
| Outcome parameters | Primary enucleation | Globe salvage group | Secondary enucleation | |||
|---|---|---|---|---|---|---|
| A. Primary outcome Response to treatment Recurrence B. Secondary outcome Complications 1 Implant exposure 2 Systemic metastasis 3 Death |
Complete- 11 01 01 Nil Nil |
Complete- 23 No response- 03 05 0 01 01 |
Complete- 06 Nil Nil Nil Nil |
Complete is defined as complete response to the treatment with no sign of recurrence at 12 months
The overall globe salvage rate was 54.76% as we could save 23 eyes out of 42. In the globe salvage group, the salvage rate was 76.6% as 23 eyes were saved out of 30 eyes. In this subset of patients, one patient who had orbital retinoblastoma developed systemic metastasis during chemotherapy and later succumbed to the disease. Globe salvage rate in group D was 100% (n = 2, out of two patients) and in group E was 52.5% (n = 21, out of 40 patients).
Discussion
The management of retinoblastoma is complex and evolving, which needs a multidisciplinary treatment approach. Few years back, the globe salvage rate in retinoblastoma was dismal and most of the time, the eyes to be enucleated to save life.[15,16] Over the period of time, the globe preservation rate has improved even in advanced retinoblastoma due to inclusion of intra-arterial chemotherapy and plaque brachytherapy in the treatment armamentarium of retinoblastoma.[17] In today’s scenario, most of the parents prefer globe salvage over enucleation when given a choice as they are aware that debilitating procedures like enucleation can affect the mental well-being of their children due to minimal social acceptance.[18] However, the risk of metastasis and recurrence is associated with multiple sessions of systemic chemotherapy and intra-arterial chemotherapy. If the patient does not respond to the treatment modality which was initiated to salvage the globe, the eye needs to be enucleated, and this should be explained in detail to the affected parents. The need of frequent follow-up should be emphasized upon as salvaging the globe in advanced retinoblastoma is a herculean task which involves frequent examination under anesthesia, six to nine cycles of systemic chemotherapy, and three to five sessions of IAC. This means frequent visits to the hospital and more expenditure on travel and stay, which is beyond the reach for many patients, especially in developing countries. The cost of IAC is also a major factor for families from a low socioeconomic status for not opting for this therapy. Hence, all the pros and cons of the eye salvage treatment in retinoblastoma should be analyzed and parents who show willingness to accept the challenges associated with this should be offered this treatment. The socioeconomic status of the patient, accessibility to all latest treatment modalities, and parent’s willingness to visit hospital frequently for follow-up should be taken into account before considering eye globe salvage in advanced retinoblastoma. The mean age of the study population was 13 months. As per the study conducted by Shields et al.,[5] Arora et al.,[19] and Gupta et al.,[20] 90% of cases were diagnosed before 5 years of age. In the current study, female children were found to be affected more as the female: male ratio was 23:19. This is not in concordance with various studies which reported that retinoblastoma does not have any gender predilection.[20,21] Maximum patients in the current study had unilateral presentation as 40 patients were found to have tumor in one eye. Bilateral presentation was found in two patients. This is in accordance with Gupta et al.[20] and Rodriguez-Galindo et al.[22] as they also reported that maximum cases in their study had unilateral presentation. In our study, the globe salvage rate was 100% in group D and 52.5% in group E. Intra-arterial chemotherapy was the preferred treatment modality as maximum cases were unilateral and due to easily availability of this treatment modality at our center. Treatment with IAC proved very beneficial as we could salvage all eyes in group D and 23 eyes out of 30 eyes in group E. This is in conformity with the studies of Munier[23] and Ong et al.,[24] which also reported that the outcome with IAC was good as the globe salvage rate was high with minimal systemic complications in both group D and group E. In their study, the globe salvage rate was 91% with IAC and intravitreal chemotherapy. In another study done by Kaliki et al.,[25] the overall globe salvage rate was 51% (37 eyes). They reported 60% globe salvage in group D and 40% in group E. Shields et al.[2,4] reported a globe salvage rate of 94% in group D and 36% in group E.
In our study, We started systemic chemotherapy in 03 patients as in remaining 27 patients, intra-arterial chemotherapy was administered as intra-arterial chemotherapy facility was available at our cente and this treatment was provided(IAC) free of cost to all our patients. Therefore, only in 03 patients we advocated systemic chemotherapy as these patients did not fit into the criteria for starting IAC. We managed to salvage one eye globe by administering systemic chemotherapeutic drugs in this group. One patient who had orbital retinoblastoma at presentation died during the course of chemotherapy.
In a study of 64 group D eyes by Fabian et al.,[26] primary IV chemotherapy with appropriate adjuvant treatment achieved globe salvage in 63% cases with no evidence of metastasis or death.
The carboplatin, vincristine, and etoposide (CVE) protocol for chemoreduction with focal therapy continues to be the conventional first-line treatment worldwide. Amin et al.[27] also reported that with systemic chemotherapy, they managed to salvage the globe in nine out of 20 eyes (45%) with group D retinoblastoma.
In the current study, the rate of primary enucleation was less in advanced retinoblastoma due to availability of all latest treatment modalities at this center. This is not in accordance with Mallipatna et al.,[28] which reported 100% rate of primary enucleation in advanced retinoblastoma.
In our study, we found recurrence of the disease in six cases. Recurrence was found 2–3 months after regression of the tumor, and the main cause of recurrence was reactivation of vitreous and subretinal seeds as retinoblastoma spreads through seeds. The intravitreal melphalan in these cases proved to be very effective in controlling seeds, and we could salvage the globe in these cases. Munier et al.[29] also reported that intravitreal melphalan was an effective treatment strategy and improved the globe salvage rate in group D and group E retinoblastoma. They also stated that intravitreal melphalan is an effective treatment, which has greatly improved the overall survival of group D eyes. Amin et al.[27] communicated in their study that the globe salvage rate increased by 15% by using intravitreal melphalan, as they could save three out of four eyes which developed recurrence.
Histopathology of eyes in the primary enucleation group showed high-risk features in three eyes (25%) as the retrolaminar part of the optic nerve was found invaded, which required adjuvant chemotherapy. The study done by Yousef et al.[30] revealed high-risk features in 11 eyes out of 50 (21%) in group E. These findings were seen in contrast to the histopathologic finding of secondary enucleated eyes. This group included 6 eyes, out of which two eyes (4.76%) showed high-risk features requiring adjuvant chemotherapy. The reason was the possible downstaging of histopathology due to chemotherapy. A retrospective study by Zhao et al.[31] showed concern regarding the possible risk of metastases secondary to downstaging for group E eyes. In our follow-up of 1 year, metastasis was found in one case.
At present, there are no standard treatment protocols for the management of advanced retinoblastoma as its management is perplexing and tricky. The outcomes of our study are heartening as our institutional concocted treatment protocol delivered good results since we managed to save all eyes with group D and 21 eyes with group E retinoblastoma. Our aim was to salvage the globe in advanced retinoblastoma without putting the patient at the risk of recurrence and systemic metastasis, which we managed to achieve among our clientele in the current study. This study has shown that chemoreduction along with focal therapy may circumvent the need for enucleation in advanced retinoblastoma without endangering the life of patients. We advocate this treatment to be followed at a center where in addition to availability of latest treatment modalities, access to positron emission tomography and in-house facility for review by pediatrician, interventional radiologist, and radiation oncologist should be present.
The main limitation of the study was its small sample size as we could treat 42 patients over a period of 4 years at our institute. The possible reason is the rarity of this disease in the Indian subcontinent. Secondly, it was a single-center study, and hence, the outcomes of the study may not be true representative of the entire population. Therefore, a study with a larger population and collaboration of multiple tertiary care centers is required to validate the outcome and reduce bias in interpretating the outcomes in advanced retinoblastoma.
Conclusion
Challenges in treating advanced retinoblastoma can be overcome by adopting a holistic and customized approach. The eye can be salvaged in this subset of patients with less risk of recurrence and systemic metastasis. Treatment with IAC using triple drugs has shown promising results and favorable outcome in this study. The need of frequent follow-up for patients who are on systemic chemotherapy and IAC should be stressed upon by the treating physician to detect recurrence and metastasis at the earliest.
Financial support and sponsorship:
Nil.
Conflicts of interest:
There are no conflicts of interest.
References
- 1.Shields CL, Mashayekhi A, Au AK, Czyz C, Leahey A, Meadows AT, et al. The international classification of retinoblastoma predicts chemoreduction success. Ophthalmology. 2006;113:2276–80. doi: 10.1016/j.ophtha.2006.06.018. [DOI] [PubMed] [Google Scholar]
- 2.Shields CL, Lally SE, Leahey AM, Jabbour PM, Caywood EH, Schwendeman R, et al. Targeted retinoblastoma management: When to use intravenous, intra-arterial, periocular, and intravitreal chemotherapy. Curr Opin Ophthalmol. 2014;25:374–85. doi: 10.1097/ICU.0000000000000091. [DOI] [PubMed] [Google Scholar]
- 3.Manjandavida FP, Honavar SG, Reddy VA, Khanna R. Management and outcome of retinoblastoma with vitreous seeds. Ophthalmology. 2014;121:517–24. doi: 10.1016/j.ophtha.2013.09.011. [DOI] [PubMed] [Google Scholar]
- 4.Shields CL, Manjandavida FP, Lally SE, Pieretti G, Arepalli SA, Caywood EH, et al. Intra-arterial chemotherapy for retinoblastoma in 70 eyes: outcomes based on the international classification of retinoblastoma. Ophthalmology. 2014;121:1453–60. doi: 10.1016/j.ophtha.2014.01.026. [DOI] [PubMed] [Google Scholar]
- 5.Shields CL, Shields JA. Retinoblastoma management: Advances in enucleation, intravenous chemoreduction, and intra-arterial chemotherapy. Curr Opin Ophthalmol. 2010;21:203–12. doi: 10.1097/ICU.0b013e328338676a. [DOI] [PubMed] [Google Scholar]
- 6.Chawla B, Hasan F, Azad R, Seth R, Upadhyay AD, Pathy S, et al. Clinical presentation and survival of retinoblastoma in Indian children. Br J Ophthalmol. 2016;100:172–8. doi: 10.1136/bjophthalmol-2015-306672. [DOI] [PubMed] [Google Scholar]
- 7.Shields CL, Jorge R, Say EA, Magrath G, Alset A, Caywood E, et al. Unilateral retinoblastoma managed with intravenous chemotherapy versus intra-arterial chemotherapy: Outcomes based on the international classification of retinoblastoma. Asia Pac J Ophthalmol. 2016;5:97–103. doi: 10.1097/APO.0000000000000172. [DOI] [PubMed] [Google Scholar]
- 8.Shields CL, Manjandavida FP, Arepalli S, Kaliki S, Lally SE, Shields JA. Intravitreal melphalan for persistent or recurrent retinoblastoma vitreous seeds: Preliminary results. JAMA Ophthalmol. 2014;132:319–25. doi: 10.1001/jamaophthalmol.2013.7666. [DOI] [PubMed] [Google Scholar]
- 9.Honavar SG, Singh AD, Shields CL, Demirci H, Smith AF, Shields JA. Post-enucleation prophylactic chemotherapy in high-risk retinoblastoma. Arch Ophthalmol. 2002;120:923–31. doi: 10.1001/archopht.120.7.923. [DOI] [PubMed] [Google Scholar]
- 10.Suzuki S, Yamane T, Mohri M, Kaneko A. Selective ophthalmic arterial injection therapy for intraocular retinoblastoma: The long-term prognosis. Ophthalmology. 2011;118:2081–7. doi: 10.1016/j.ophtha.2011.03.013. [DOI] [PubMed] [Google Scholar]
- 11.Lee V, Hungerford JL, Bunce C, Ahmed F, Kingston JE, Plowman PN. Globe conserving treatment of the only eye in bilateral retinoblastoma. Br J Ophthalmol. 2003;87:1374–80. doi: 10.1136/bjo.87.11.1374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kaliki S, Shields CL, Rojanaporn D, Al-Dahmash S, McLaughlin JP, Shields JA, et al. High-risk retinoblastoma based on international classification of retinoblastoma: Analysis of 519 enucleated eyes. Ophthalmology. 2013;120:997–1003. doi: 10.1016/j.ophtha.2012.10.044. [DOI] [PubMed] [Google Scholar]
- 13.Fabian ID, Stacey AW, Johnson KC, Chowdhury T, Duncan C, Reddy MA, et al. Primary enucleation for group D retinoblastoma in the era of systemic and targeted chemotherapy: The price of retaining an eye. Br J Ophthalmol. 2018;102:265–71. doi: 10.1136/bjophthalmol-2017-310624. [DOI] [PubMed] [Google Scholar]
- 14.Wilson MW, Qaddoumi I, Billups C, Haik BG, Rodriguez Galindo C. A clinicopathological correlation of 67 eyes primarily enucleated for advanced intraocular retinoblastoma. Br J Ophthalmol. 2011;95:553–8. doi: 10.1136/bjo.2009.177444. [DOI] [PubMed] [Google Scholar]
- 15.Chen Q, Zhang B, Dong Y, Mo X, Zhang L, Huang W, et al. Comparison between intravenous chemotherapy and intra-arterial chemotherapy for retinoblastoma: A meta- analysis. BMC Cancer. 2018;18:486. doi: 10.1186/s12885-018-4406-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Abramson DH, Marr BP, Francis JH, Dunken IJ, Fabius AW, Brodie SE, et al. Simultaneous bilateral ophthalmic artery chemosurgery for bilateral retinoblastoma. PLoS One. 2016;11:6. doi: 10.1371/journal.pone.0156806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chen M, Jiang H, Zhang J, Shen G, Jiang Y, Li H, et al. Outcome of intra-arterial chemotherapy for retinoblastoma and its influencing factors: A retrospective study. Acta Ophthalmol. 2017;95:613–8. doi: 10.1111/aos.13333. [DOI] [PubMed] [Google Scholar]
- 18.Ghassemi F, Gjanaati H, Karkhaneh R, Boujabadi L, Tabatabaie SZ, Rajabi MT. Outcome of retinoblastoma following limited session of intra-arterial chemotherapy in Iran. Iran J Radiol. 2014;11:16958. doi: 10.5812/iranjradiol.16958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Arora RS, Eden T, Kapoor G. Epidemiology of childhood cancer in India. Indian J Cancer. 2009;46:264–73. doi: 10.4103/0019-509X.55546. [DOI] [PubMed] [Google Scholar]
- 20.Gupta N, Pandey A, Dimri K, Prinja S. Epidemiological profile of retinoblastoma in North India: Implications for primary care and family physicians. J Family Med Prim Care. 2020;9:2843–8. doi: 10.4103/jfmpc.jfmpc_265_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Padma M, Kumar N, Nesargi PS, Kumari BSA, Appaji L, Viswanathan A. Epidemiology and clinical features of retinoblastoma; A tertiary care center’s experience in India. South Asian J Cancer. 2020;9:56–8. doi: 10.4103/sajc.sajc_89_19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rodriguez-Galindo C, Wilson MW, Chantada G, Fu L, Quaddoumi I, Antoneli C, et al. Retinoblastoma: One world, one vision. Pediatrics. 2008;122:763–70. doi: 10.1542/peds.2008-0518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Munier FL. Classification and management of seeds in retinoblastoma. Ellsworth Lecture Ghent August 24th 2013. Ophthalmic Genet. 2014;35:193–207. doi: 10.3109/13816810.2014.973045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ong SJ, Chao AN, Wong HF, Liou KL, Kao LY. Selective ophthalmic arterial injection of melphalan for intraocular retinoblastoma: A 4-year review. Jpn J Ophthalmol. 2015;59:109–17. doi: 10.1007/s10384-014-0356-y. [DOI] [PubMed] [Google Scholar]
- 25.Kaliki S, Mittal P, Mohan S, Chattannavar G, Jajapuram SD, Mohamed A, et al. Bilateral advanced (group D or group E) intraocular retinoblastoma: Outcomes in 72 Asian Indian patients. Eye (Lond) 2019;33:1297–304. doi: 10.1038/s41433-019-0409-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fabian ID, Stacey AW, Johnson KP, Onadim Z, Chowdhury T, Duncan C, et al. Primary intravenous chemotherapy for group D retinoblastoma: A 13-year retrospective analysis. Br J Ophthalmol. 2017;101:82–8. doi: 10.1136/bjophthalmol-2016-309710. [DOI] [PubMed] [Google Scholar]
- 27.Amin S, Aljboor M, Toro MD, Rejdar R, Nowomiesjska K, Nazzal R, et al. Management and outcomes of unilateral group D tumors in retinoblastoma. Clin Ophthalmol. 2021;15:65–72. doi: 10.2147/OPTH.S282741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mallipatna AC, Sutherland JE, Gallie BL, Chan H, Heon E. Management and outcome of unilateral retinoblastoma. J AAPOS. 2009;13:546–50. doi: 10.1016/j.jaapos.2009.09.004. [DOI] [PubMed] [Google Scholar]
- 29.Munier FL, Soliman S, Moulin AP, Gaillard MC, Balmer A, Beck-Popovic M. Profiling safety of intravitreal injections for retinoblastoma using an anti-reflux procedure and sterilisation of the needle track. Br J Ophthalmol. 2012;96:1084–7. doi: 10.1136/bjophthalmol-2011-301016. [DOI] [PubMed] [Google Scholar]
- 30.Yousef YA, Hajja Y, Nawaiseh I, Mehyar M, Sultan I, Deebajah R, et al. A histopathologic analysis of 50 eyes primarily enucleated for retinoblastoma in a tertiary cancer center in Jordan. Turk Patoloji Derg. 2014;30:171–7. doi: 10.5146/tjpath.2014.01260. [DOI] [PubMed] [Google Scholar]
- 31.Zhao J, Dimaras H, Massey C, Xu X, Huang D, Li B, et al. Pre-enucleation chemotherapy for eyes severely affected by retinoblastoma masks risk of tumor extension and increases death from metastasis. J Clin Oncol. 2011;29:845–51. doi: 10.1200/JCO.2010.32.5332. [DOI] [PubMed] [Google Scholar]


