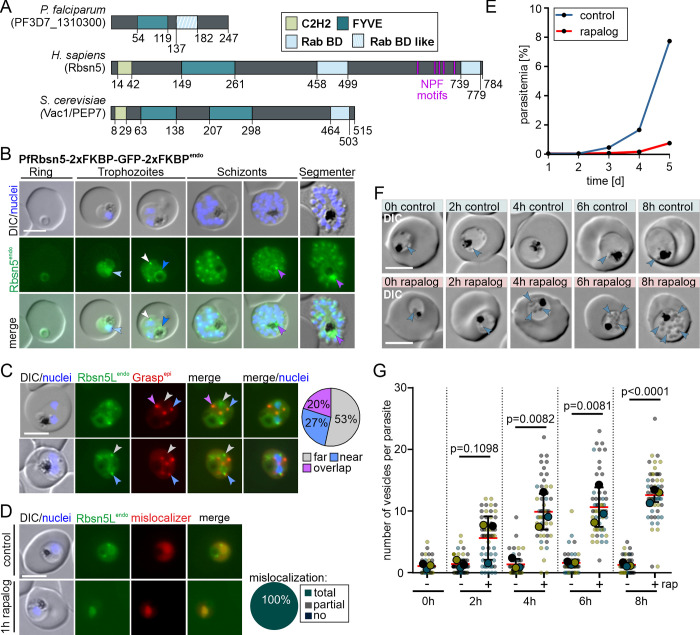
Fig 1. Conditional Inactivation of PfRbsn5L leads to vesicles in the parasite and parasite death.
(A) Comparison of domain architecture of the putative PfRbsn5L, H. sapiens HsRbsn5, and S. cerevisiae VAC1/PEP7. BD, binding domain C2H2. (B) Live-cell microscopy images of the indicated stages of PfRbsn5L-2xFKBP-GFP-2xFKBPendo parasites (white arrow, nucleus-proximal PfRbsn5Lendo foci; light blue arrow, faint dispersed signal in the nucleus; dark blue arrow, signal at the food vacuole; purple arrow, intense focus at the food vacuole in schizont stage parasites). (C) Live-cell microscopy images of PfRbsn5L-2xFKBP-GFP-2xFKBPendo parasites, co-expressing Graspepi. Arrows show PfRbsn5Lendo foci close to nuclei and are color coded based on overlap with the closest GRASP focus. Overlap: full overlap; near: less than GRASP focus diameter apart, far: more than one focus diameter apart. Pie shows proportion of these foci (n = 45 with cells from 3 independent imaging sessions). (D) Live-cell microscopy images of PfRbsn5L (PfRbsn5L-2xFKBP-GFP-2xFKBPendo+ nmd3’NLS-FRB-mChepi parasites) using a nuclear mislocalizer (mislocalizer) [30] 1 h after induction of knock-sideway (1 h rapalog) compared to the control (control). Knock-sideway was classified (pie chart) as complete (no signal detected outside the nucleus), partial (signal in the nucleus but also at original site), or absent (no) mislocalization in n = 30 parasites from 2 independent experiments. (E) Flow cytometry-based growth curve over 2.5 growth cycles of PfRbsn5L knock-sideways (rapalog) compared to the control parasites. One representative of n = 3 independent experiments, all replicas shown in (S1F Fig). (F) Representative DIC live-cell images of parasites 0 h, 2 h, 6 h, and 8 h after induction of knock-sideways of PfRbsn5L (+ rapalog) compared to control. Blue arrows, vesicular structures. (G) Quantification of number of vesicles in synchronous trophozoites 0 h, 2 h, 6 h, and 8 h after induction of PfRbsn5L knock-sideways. Data shown as superplot [77] from n = 3 independent experiments (individual experiments: blue, yellow, and black with 147, 176, and 144 parasites (small dots), respectively; average of each experiment as large dot); two-tailed unpaired t test; red lines, mean; black lines, error bar (SD); p-values indicated. Scale bars, 5 μm and 1 μm in the magnifications. Nuclei were stained with DAPI. DIC, differential interference contrast; endo, endogenous; epi, episomal; Rbsn5Lendo, 2xFKBP-GFP-2xFKBP-tagged Rbsn5L expressed from endogenous locus. The data underlying this figure can be found in S1 Data.