Figure 4. Glycosylation impacts therapeutic efficacy of biologics beyond protein therapeutics.
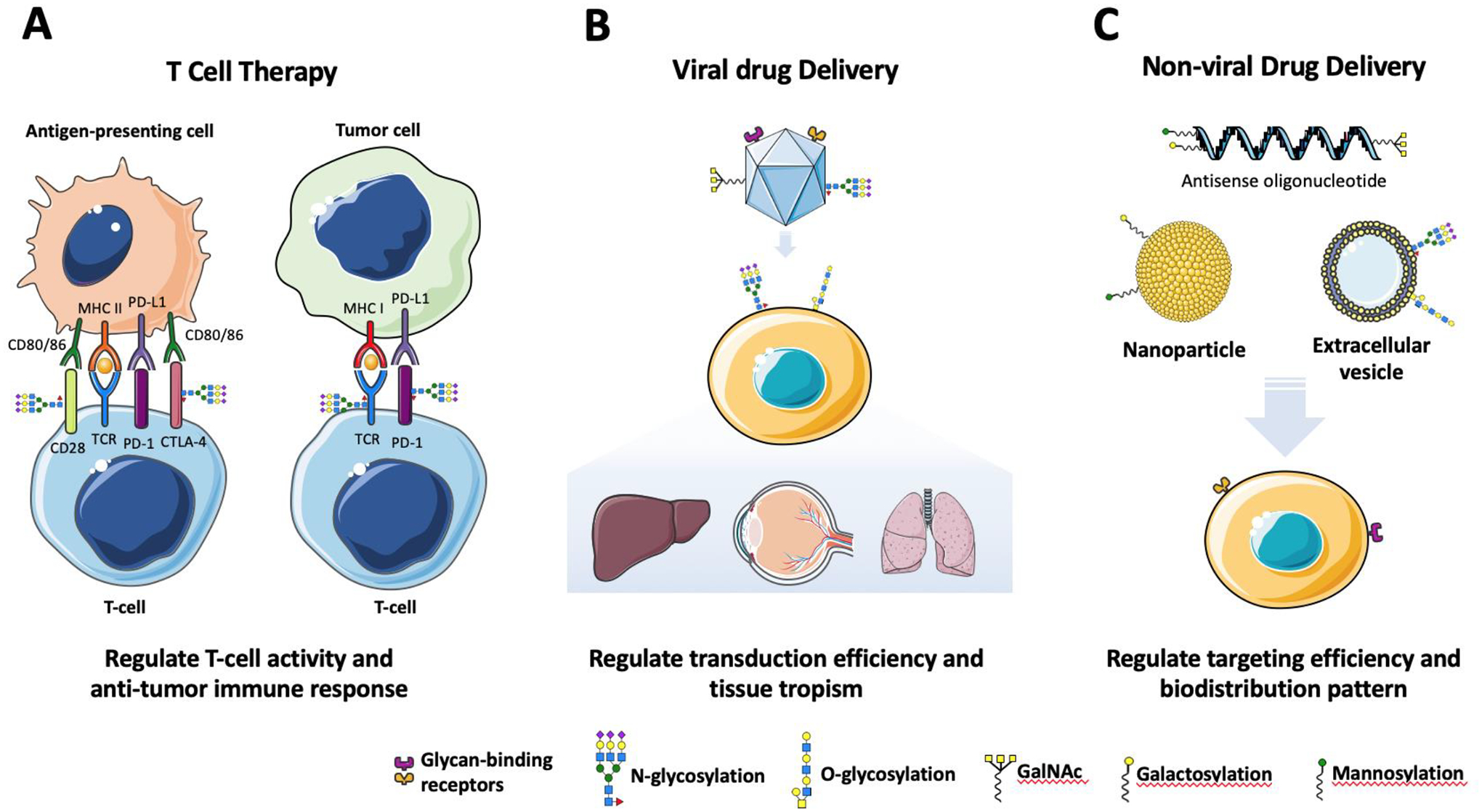
(A) Anticancer T-cell therapies rely on interactions between the T-cell and the tumor cell or antigen presenting cell. Some of these important interactions include CD28⬄CD80/CD86 which positively regulates T-cell activation and promote downstream anti-tumor activity, while PD1⬄PDL1 and CTLA-4⬄CD80/CD86 negatively regulate T-cell activation and reduce anti-tumor activity. N-linked glycan heterogeneity on these interacting proteins significantly impacts their binding affinity, expression level and cellular localization and therefore play a role in ensuring the therapeutic efficacy of such cell-based treatment modalities. (B) The presence/absence and identity of N-linked glycans on AAV capsid proteins can affect host cell infectivity, vector yield and immune response. Furthermore, the primary receptors of all AAV serotypes are O-linked and N-linked glycans such as terminal sialic acid and terminal galactose residues found on the host cell surface. N- and O-linked glycosylation, therefore, are key factors that modulate AAV transduction efficiency and tissue tropism. (C) Because different cell types usually express a distinct array of glycan receptors, conjugation of glycan structures to naked oligonucleotide-based drugs and delivery platforms such as nanoparticles and extracellular vesicles can improve targeting efficiency as well as modulate biodistribution in vivo.
