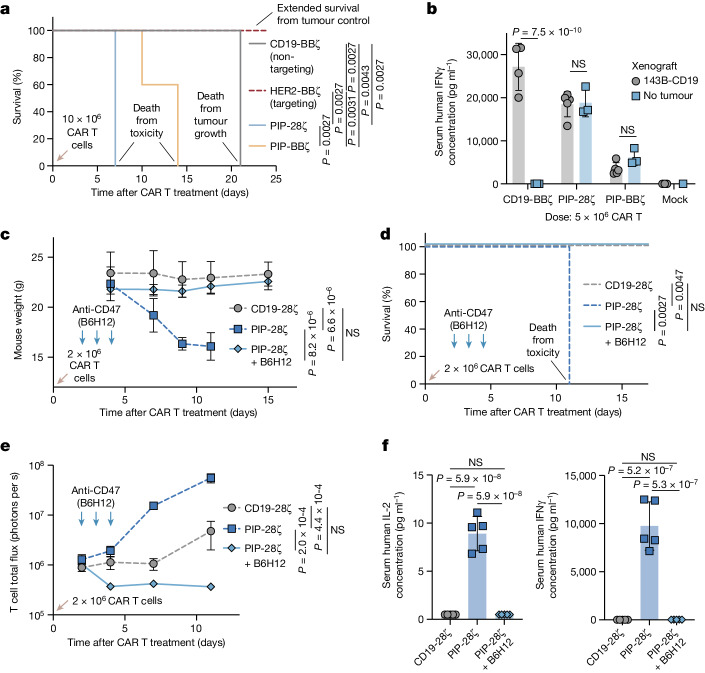
Fig. 3. Anti-CD47 therapy can be used as a safety switch.
a, The survival of 143B tumours after treatment with CD19-BBζ (non-targeting), HER2-BBζ (targeting), PIP-28ζ or PIP-BBζ CAR T cells. n = 5 mice per arm. b, Human IFNγ in the blood of mice with or without 143B-CD19 tumours, treated with CD19-BBζ, PIP-28ζ or PIP-BBζ CAR T cells on day 4, as determined using LEGENDPlex quantitative flow cytometry. Data are mean ± s.d. of n = 5 (PIP-28ζ or PIP-BBζ with 143B-CD19 tumour), n = 4 (CD19-BBζ with 143B-CD19 tumours), n = 3 (mock with 143B-CD19 tumour and CD19-BBζ, PIP-28ζ or PIP-BBζ without tumours) and n = 1 (mock without tumour) mice. c, Mouse weights after PIP-28ζ CAR T cell treatment with or without B6H12. Data are mean ± s.d. of n = 5 mice per arm. Representative of two independent experiments. d, The survival of mice treated with PIP-28ζ CAR T cells with or without B6H12. n = 5 mice per arm. Representative of two independent experiments. e, T cell BLI after treatment with PIP-28ζ CAR T cells with or without B6H12. Data are mean ± s.e.m. of n = 5 mice per arm. f, IL-2 (left) and IFNγ (right) in the blood of mice treated with PIP-28ζ CAR T cells with or without B6H12 on day 4, as determined using LEGENDPlex quantitative flow cytometry. Data are mean ± s.d. of n = 5 mice. Statistical analysis was performed using log-rank Mantel–Cox tests (a and d), unpaired two-tailed Student’s t-tests (b), two-way ANOVA (c and e) and one-way ANOVA with Tukey’s multiple-comparison test (f).