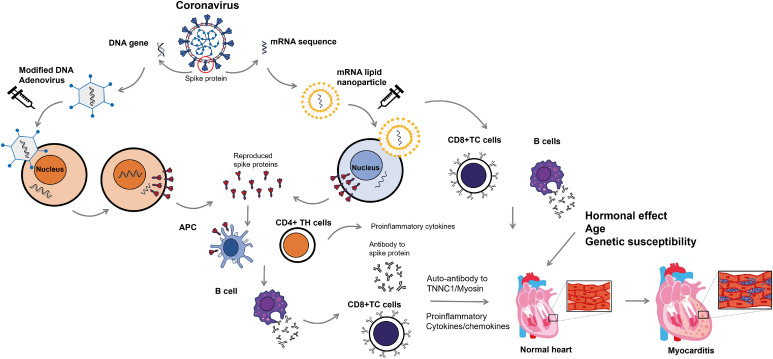
Figure 1. Proposed pathophysiologic mechanism of coronavirus disease 2019 vaccine-related myocarditis. Vaccines for severe acute respiratory syndrome coronavirus-2 use the viral spike protein as their target. Adenoviral vaccines use a modified form of DNA adenovirus to elicit an immune response to the viral spike proteins. Upon injection, the DNA is released into the cytoplasm and later migrates into the cell nucleus without being incorporated into the cellular DNA. DNA produces mRNA that codes for viral spike proteins. In contrast, mRNA vaccines use mRNA for viral spike protein that is included in lipid nanoparticles. The mRNA then migrates back into the cytoplasm. After injection of these vaccines, the mRNA inserts itself into the cytoplasm, where it synthesizes the viral spike proteins via interaction with ribosomes. Viral spike proteins can elicit an immune response from the host’s helper T cells (CD4+ TH) and APC, resulting in the production of antibodies against viral spike proteins by B cells. Infected cells are attacked by cytotoxic T cells (CD8+ TC) coated with antibodies against viral spike proteins. It is important to note that antibodies against spike proteins may also have cross-reactivity with TNNC1 and myosin, potentially leading to an attack on the myocardium. Increased immune responses, including elevated serum concentrations of proinflammatory cytokines and chemokines, can enhance the attack. In females, sex hormones such as estrogen inhibit T cell activity and reduce the immune response. Additionally, genetic factors may contribute to the pathogenesis of myocarditis.
APC = antigen-presenting cells; TC = cytotoxic T cells; TH = helper T cells; TNNC1 = cardiac troponin C1.