Abstract
Background
There is no established standard 3rd line treatment for patients with advanced non-small cell lung cancer (NSCLC). Although cytotoxic chemotherapeutic agents that are not used as 1st or 2nd line treatment are administrated as 3rd line treatment, their anti-tumor efficacy is insufficient. Anti-programmed death ligand-1 (PD-L1)/programmed death-1 (PD1) treatment is more effective and less toxic than chemotherapy in anti-PD-L1/PD-1 treatment-naïve patients with NSCLC. Therefore, anti-PD-L1/PD-1 therapy is considered an appropriate 3rd line treatment. However, the anti-tumor efficacy is limited in patients previously treated with anti-PD-L1/PD-1 antibody. Today, new drugs are needed to increase the efficacy of anti-PD-L1/PD-1 antibodies.
Methods
This open-label, single-arm, investigator-initiated phase II study is designed to evaluate combination treatment of nivolumab and TM5614, a plasminogen activator inhibitor (PAI-1) inhibitor as 3rd or more line treatment in NSCLC patients who underwent standard treatment. The primary endpoint is the objective response rate and the secondary endpoints are progression-free survival (PFS), overall survival (OS), duration of response (DOR) and safety. Recruitment began in September 2023 and is expected to continue for approximately three years.
Discussion
Currently, there is no standard 3rd line treatment for advanced NSCLC, and we hope that the findings of this study will facilitate more effective treatments in this setting. Ethics and dissemination: the study protocol conformed to the ethical principles outlined in the Declaration of Helsinki. All patients will provide written informed consent prior to enrollment. Results will be published in a peer-reviewed publication.
Trial Registration
This study is registered to Japan Registry of Clinical Trials with number: jRCT2061230039 (19/July/2023).
Keywords: Non-small cell lung cancer (NSCLC), anti-programmed death-1 antibody, plasminogen activator inhibitor-1 (PAI-1), TM5614, nivolumab
Introduction
Lung cancer is the most common cause of cancer-related death (1), and is classified into two broad histological types: non-small cell lung carcinoma (NSCLC) and small-cell lung carcinoma. A standard treatment for patients with advanced NSCLC, a performance status of 0–1, and no driver mutations is platinum-based doublet chemotherapy and anti-programmed death ligand-1 (PD-L1)/programmed death-1 (PD1) antibody (2). However, the 2-year progression-free survival (PFS) rate is only 22%, and few patients show a complete response (CR) (3). Although platinum doublet chemotherapy with anti-PD-1 antibody and anti-cytotoxic T lymphocyte-associated antigen 4 (CTLA-4) antibody is available, no remarkable improvement in treatment outcomes has been observed (4,5). The standard 2nd line treatment is docetaxel and ramucirumab, whose PFS is reported to be only 4.5 months, hence their efficacy is insufficient (6). Additionally, a standard 3rd line treatment has not yet been established.
Currently, single-agent chemotherapeutic drugs, including nab-paclitaxel, which are not used until 2nd line treatment, are administrated as 3rd line treatment, but their response rate ranges from 5% to 20%, and the incidence of severe adverse events ranges from 30–50% (7-13). In contrast, a phase III study showed that the response rates of anti-PD-1 antibodies, such as nivolumab, pembrolizumab, and docetaxel, were approximately 20% (for the two former drugs) and 9%, respectively, and the efficacy of anti-PD-1 antibody was superior to that of docetaxel in anti-PD-1 treatment-naive patients. Furthermore, the incidence of severe adverse events is lower with anti-PD-1 treatment than with docetaxel treatment (14,15). Therefore, anti-PD-1 therapy is an appropriate candidate for 3rd line treatment. However, the response rate to nivolumab was only 8.5% in patients previously treated with anti-PD-1/PD-L1 antibody (16). Thus, to improve the anti-tumor efficacy of anti-PD-1/PD-L1 treatment in patients previously treated with anti-PD-1/PD-L1, new drugs, such as anti-CTLA-4 antibody, are being developed. However, anti-CTLA-4 antibodies are associated with increasing lethal immune-related side effects, such as pneumonitis and hypercytokinemia, as well as medical costs. Therefore, new treatment agents with fewer side effects and lower medical costs are required to increase the efficacy of anti-PD-1/PD-L1 antibodies in patients previously treated with anti-PD-1/PD-L1 antibodies.
Plasminogen activator inhibitor-1 (PAI-1) is a glycoprotein with a molecular weight of 47-kDa produced by vascular endothelial cells, macrophages, and platelets and is an inhibitor of the fibrinolytic system (17). In contrast, PAI-1 is reported to be involved in the proliferation and apoptosis of cancer cells and tumor angiogenesis, resulting in tumor progression in lung, breast, cervical cancers and malignant mesothelioma (18-20). In addition, we showed that PAI-I is involved in chemotherapeutic resistance through the activation of cancer-associated fibroblasts and epithelial-mesenchymal transition in lung cancer cells (21). We have also found that PAI-1 is associated with resistance of lung cancer cells to PD-1 antibody treatment.
TM5614, a PAI-1 inhibitor, was selected from more than 1,400 novel derivatives explored by in silico drug discovery based on the crystal structure of human PAI-1. TM5614 is a small-molecule drug developed in academia (Tohoku University) through discovery, optimization, good non-clinical laboratory practice studies, and good manufacturing practice (GMP) regarding compliant synthesis and formulation (22,23). TM5614 has been found to be well tolerated in Phase I studies in healthy subjects. In addition, the antitumor efficacy of the combination treatment with TM5614 and an anti-PD-1 antibody has been observed to be higher than that of an anti-PD-1 antibody treatment in an in vivo study using a mouse lung cancer model. Our studies revealed that TM5614 improved the tumor immune-microenvironment, such as an increase in cytotoxic T cells and a decrease in tumor-associated macrophages and PD-L1 expression of cancer cells. Furthermore, a phase II study examining the efficacy of TM5614 combined with a tyrosine kinase inhibitor in patients with chronic myelogenous leukemia was completed (24) and a phase III study is ongoing. In addition, a phase II study was conducted to investigate the combination treatment with nivolumab and TM5614 in patients with melanoma (jRCT2021210029). To date, no safety issues have been reported. Based on these observations, TM5614 is being used in addition to nivolumab in the present study. We present this article in accordance with the SPIRIT reporting checklist (available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-1858/rc).
Methods
Study design and objective
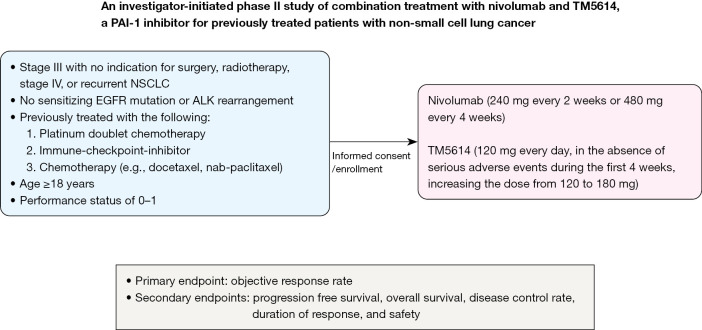
This open-label, single-arm, investigator-initiated phase II study will evaluate the efficacy and safety of combination treatment with nivolumab and TM5614, a PAI-1 inhibitor as 3rd or more line therapy for patients with NSCLC who were previously treated with platinum doublet chemotherapy, anti-PD-1/PD-L1 treatment, and one more cytotoxic chemotherapy. Figure 1 shows a flowchart of the study. Six hospitals have agreed to participate in this study.
Figure 1.
Study flow chart. PAI-1, plasminogen activator inhibitor-1; NSCLC, non-small cell lung cancer; EGFR, epidermal growth factor receptor; ALK, anaplastic lymphoma kinase.
Endpoints
The primary endpoint is the objective response rate. The secondary endpoints are PFS, overall survival (OS), disease control rate, duration of response (DOR), and safety. The exploratory endpoint is the optimal cut-off value of pretreatment plasma PAI-1 levels to discriminate radiological tumor response or disease control from disease progression.
Key eligibility criteria
Key inclusion and exclusion criteria are listed in Tables 1,2.
Table 1. Key inclusion criteria.
| Inclusion criteria |
| (I) Age of ≥18 years at the time of informed consent |
| (II) Provision of written informed consent |
| (III) Histologically confirmed stage III with no indication for curative surgery, radiotherapy, stage IV, or recurrent NSCLC |
| (IV) No sensitizing epidermal growth factor receptor mutations or anaplastic lymphoma kinase rearrangements in patients with non-squamous carcinoma |
| (V) No or unknown ROS-1 rearrangement, BRAF (V600E) gene mutation, MET exon 14 skipping mutation, RET rearrangement, or NTRK rearrangement |
| (VI) Disease progression during or after the most recent treatment and history of treatment |
| (VII) Patients who received the following treatments |
| • Platinum doublet chemotherapy |
| • Immune checkpoint inhibitors (e.g., nivolumab, pembrolizumab, atezolizumab, nivolumab, and ipilimumab) |
| • Chemotherapy (e.g., pemetrexed, docetaxel, nab-paclitaxel, S-1) |
| (VIII) Measurable lesions based on RECIST Ver.1.1 |
| (IX) Eastern Cooperative Oncology Group performance status of 0–1 |
| (X) Estimated life expectancy of >3 months |
| (XI) Adequate organ function within 7 days prior to registration |
NSCLC, non-small cell lung cancer; RECIST, Response Evaluation Criteria in Solid Tumors.
Table 2. Key exclusion criteria.
| Exclusion criteria |
| (I) Active autoimmune disease that has required systemic treatment using corticosteroids or other immunosuppressive medication |
| (II) History of serious immune-related adverse events caused by anti-PD-1/anti-PD-L1 antibodies |
| (III) Patients receiving continuous systemic administration (oral or intravenous) of steroids or other immunosuppressive drugs exceeding 10 mg/day of prednisolone equivalent |
| (IV) Multiple cancers |
| (V) Central nervous system metastases (symptomatic or requiring treatment) |
| (VI) Carcinomatous meningitis |
| (VII) Evidence of interstitial pneumonia |
| (VIII) Uncontrollable pleural effusion, ascites, or pericardial fluid |
| (IX) Underwent radiotherapy within 2 weeks before the first nivolumab and TM5614 |
| (X) Evidence of severe or uncontrolled systemic disease |
PD-1, programmed death-1; PD-L1, programmed death ligand 1.
Estimation of sample size
The response rate to cytotoxic chemotherapy used as 3rd or more line treatment has been previously reported to be approximately 5–20% (7-13). All patients to be enrolled in this study received anti-PD-L1/PD-1 antibody treatment previously. Thus, the response rate in our study is likely to be lower than that of a previous study. Additionally, a previous study showed that the response rate to nivolumab in patients previously treated with an anti-PD-L1/PD-1 antibody was 8.5% (16). In that study, patients who achieved disease control with anti-PD-L1/PD-1 antibody for 6 months and whose last administration of anti-PD-L1/PD-1 antibody was more than 60 days earlier were enrolled. These criteria were excluded from our study. Thus, we set the response rate threshold at 6.0%. The expected response rate was set at 20% based on the efficacy of TM5614 and the previously reported response rate of cytotoxic chemotherapy used as third or further lines (9,11). The sample size was determined using an exact binomial test based on a threshold response rate of 6.0%, an expected response rate of 20.0% in the present study, one-sided alpha value of 0.05, and a power of 0.8. Based on these parameters, 39 patients will be enrolled in this study. We will include 39 patients in the full analysis set.
Treatment
Patients will receive intravenous nivolumab (240 mg every 2 weeks or 480 mg every 4 weeks) and oral administration of TM5614 (120 mg every day, in the absence of serious adverse events during the first 4 weeks, increasing the dose from 120 to 180 mg), until they experience radiologic disease progression, treatment-related adverse events of unacceptable severity, withdrawal of consent, or termination of treatment at the discretion of the investigator.
Patient registration
After confirming eligibility and obtaining a signed informed consent form, each patient will be registered and will receive treatment. Patient recruitment began in September 2023. It is expected to continue until December 2024. The observational period will be one year from the time of the final registration.
Follow-up and assessment
Patients must undergo various pretreatment evaluations, including a computed tomography (CT) or magnetic resonance imaging scan of the brain, CT scans of the chest and abdomen, a bone scan or positron emission tomography scan, and electrocardiography. Patients will be followed up for at least one year from the time of enrollment. Patients will undergo tumor assessments at baseline, every 4 weeks for 3 cycles, every 8 weeks after 4 cycles. Tumor response and/or radiologic disease progression will be evaluated based on RECIST Ver.1.1. (25). Adverse events are recorded using the National Cancer Institute’s Common Terminology Criteria for Adverse Events, version 5.0. (26).
Statistical analysis
The primary endpoint is the objective response rate. The objective response rate [CR + partial response (PR) proportion] will be calculated, and the one-side 95% confidence interval calculated using the Clopper-Pearson method. The secondary endpoints are PFS, OS, disease control rate, DOR, and safety. PFS is defined as the time from treatment initiation to the occurrence of death, progression of disease (PD). Overall survival (OS) is defined as the time from the initiation of treatment to death. DOR is defined as the time from the date of the first documentation of objective tumor response (CR or PR) to the first documentation of PD or to death due to any cause. The Kaplan-Meier method will be used to analyze PFS, DOR and OS. Ninety-five percent confidence intervals of survival function or median survival times will be calculated using Greenwood’s formula or Brookmeyer and Crowley’s method, respectively. The disease control rate (CR + PR + SD proportion) and 95% confidence interval will also be calculated. Adverse events observed during the protocol treatment are being summarized by type and grade. The optimal cut-off value of pretreatment serum PAI-1 level was determined through receiver operating characteristic curve analysis.
Ethics and informed consent
The trial received ethical approval from the institutional review board of Hiroshima University Hospital, Hiroshima, Japan (date of approval; 3/July/2023, the approval No. 50047) and was approved by the ethics committee of each participating institution. The study will be conducted in accordance with the Declaration of Helsinki (as revised in 2013). All patients will provide written informed consent prior to enrollment.
Trial registration
This study is registered to Japan Registry of Clinical Trials with number: jRCT2061230039 (19/July/2023).
Discussion
There has not been an established standard 3rd line treatment for advanced NSCLC, and cytotoxic chemotherapeutic agents are administered. However, their response rates range from 5% to 20% and the incidence of severe adverse events ranges from 30–50% (7-13). Therefore, more effective and safer treatments are required. Currently, platinum doublet chemotherapy, anti-PD-L1/PD-1 antibody, and cytotoxic chemotherapy including docetaxel are the standard 1st and 2nd line treatment (2). Thus, cancer cells from patients whose disease progressed during these treatments acquired resistance to chemotherapy and anti-PD-1/PD-L1 antibody treatment. PAI-1 is involved in resistance of lung cancer cells to chemotherapy (21). In addition, PAI-1 is associated with resistance to anti-PD-1 antibody treatment in lung cancer cells, and the antitumor efficacy of combination treatment with TM5614, a PAI-1 inhibitor, and an anti-PD-1 antibody was higher than that of anti-PD-1 antibody treatment in an in vivo study using mouse model. Based on these observations, TM5614, a PAI-1 inhibitor, is considered a reasonable third-line treatment drug in combination with nivolumab for 3rd line treatment after chemotherapy and anti-PD-1/PD-L1 antibody treatment.
There is a limitation in this study. This study is single arm study. Thus, we need to validate the results of this phase II study in a future trial with a larger sample to compare the treatment with the standard treatment.
To the best of our knowledge, the present study is the first clinical trial to investigate the efficacy and safety of nivolumab and TM5614, a PAI-1 inhibitor as 3rd or more line therapy for patients with advanced or recurrent NSCLC after standard treatment. We hope that these findings will facilitate a more effective and safe treatment in this setting.
Supplementary
The article’s supplementary files as
Acknowledgments
We thank Ken Masuda (Department of Respiratory Medicine, Hiroshima Prefectural Hospital, Hiroshima, Japan), Tadashi Senoo (Department of Respiratory Internal Medicine, Kure Medical Center, Kure, Japan), Ryohei Nishino (Department of Respiratory Internal Medicine, Hiroshima City Asa Citizens Hospital, Hiroshima, Japan) from the Safety Monitoring Committee. We would like to thank Editage (www.editage.com) for English language editing.
Funding: This study was supported by Renascience Inc. (to Hiroshima University Hospital).
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The trial received ethical approval from the institutional review board of Hiroshima University Hospital, Hiroshima, Japan (date of approval; 3/July/2023, the approval No. 50047) and was approved by the ethics committee of each participating institution. The study will be conducted in accordance with the Declaration of Helsinki (as revised in 2013). All patients will provide written informed consent prior to enrollment.
Footnotes
Reporting Checklist: The authors have completed the SPIRIT reporting checklist. Available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-1858/rc
Peer Review File: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-1858/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-1858/coif). T.M. reports honoraria from Chugai Pharmaceutical Co. Ltd., AstraZeneca K.K., Eli Lilly Japan K.K., MSD K.K., Taiho Pharmaceutical Co., Ltd., Ono Pharmaceutical Co., Ltd., Bristol-Myers Squibb Co. Ltd. and Kyowa Kirin Co., Ltd. outside the submitted work. T.S. reports honoraria from Chugai Pharmaceutical Co. Ltd., AstraZeneca K.K., Eli Lilly Japan K.K., MSD K.K., Taiho Pharmaceutical Co., Ltd., Ono Pharmaceutical Co., Ltd., Kyowa Kirin Co., Ltd., Novartis Pharma K.K., Merck Biopharma Co., Ltd., Janssen Pharmaceutical K.K., Amgen Inc., Daiichi-Sankyo Co., Ltd., Takeda Pharmaceutical Co., Ltd., Hisamitsu Pharmaceutical Co., Inc. and Illumina, Inc. outside the submitted work. Y.T. reports honoraria from Daiichi Sankyo Co. Ltd., AstraZeneca K.K., Chugai Pharmaceutical Co. Ltd., Kyowa Kirin Co., Ltd., TAIHO PHARMACEUTICAL Co., Ltd. and Bristol-Myers Squibb K.K. outside the submitted work, in addition to grants from Pfizer Health Research Foundation outside the submitted work. E.I. reports grants from Janssen Pharmaceutical K.K., Pfizer Japan Inc., Bristol-Myers Squibb Co. Ltd., Ono Pharmaceutical Co., Ltd. and Takeda Pharmaceutical Co., Ltd., outside the submitted work and honoraria from Chugai Pharmaceutical Co. Ltd., AstraZeneca K.K., Eli Lilly Japan K.K., Ono Pharmaceutical Co. Ltd., Takeda Pharmaceutical Co., Ltd., Bristol-Myers Squibb Co. Ltd., Pfizer Japan Inc., Janssen Pharmaceutical K.K., Novartis Pharma K.K. and Boehringer-Ingelheim Japan Inc., outside the submitted work and research material from AstraZeneca K.K. and Janssen Pharmaceutical K.K. outside the submitted work. T.K. reports honoraria from Chugai Pharmaceutical Co. Ltd., AstraZeneca K.K., Eli Lilly Japan K.K., Ono Pharmaceutical Co. Ltd., Takeda Pharmaceutical Co., Ltd., Bristol-Myers Squibb Co. Ltd., Pfizer Japan Inc., Novartis Pharma K.K., Taiho Pharmaceutical Co., Ltd., MSD K.K., Kyowa Kirin Co., Ltd., Boehringer-Ingelheim Japan Inc., Nippon Kayaku Co., Ltd., Daiichi-Sankyo Co., Ltd., Amgen Inc., Eisai Co. Ltd., Merck Biopharma Co., Ltd., Bayer Holding Ltd., Sawai Pharmaceutical Co., Ltd. outside the submitted work and grants from Chugai Pharmaceutical Co. Ltd., AstraZeneca K.K., Eli Lilly Japan K.K., Ono Pharmaceutical Co. Ltd., Bristol-Myers Squibb Co. Ltd., Pfizer Japan Inc., Taiho Pharmaceutical Co., Ltd., MSD K.K., Kyowa Kirin Co., Ltd., Daiichi-Sankyo Co., Ltd., Amgen Inc., Eisai Co. Ltd., Merck Biopharma Co., Ltd., Bayer Holding Ltd., Pfizer Japan Inc., AbbVie GK., LabCorp Japan, G.K., IQVIA Inc., Gilead Sciences, Inc. outside the submitted work, and consulting fee from Chugai Pharmaceutical Co. Ltd., AstraZeneca K.K., Ono Pharmaceutical Co. Ltd., Pfizer Japan Inc., Daiichi-Sankyo Co., Ltd., Bayer Holding Ltd., AbbVie GK. outside the submitted work. S.H. reports honoraria from Bristol-Myers Squibb Co. Ltd. outside submitted work. Y.O. reports consulting fee from Kitasato University Hospital and Association of Medical Education and Ethics outside submitted work, and honoraria for lectures of biostatistics from Tokai University, Graduate School of Medicine outside submitted work and is Data Monitoring Committee member of EVA-001-02 study sponsored by Evastem Co., Ltd., and Certified Committee member for Regenerative Medicine, Tokai University outside the submitted work. T.M. declares research grants from Astellas Pharma Inc., Daiichi Sankyo Co., Ltd., and KOWA DENTAL HEALTH K.K. and Renascience Inc. outside the submitted work, and honoraria from Renascience Inc. outside the submitted work, and stocks of Renascience Inc. N.H. reports honoraria from Chugai Pharmaceutical Co. Ltd., AstraZeneca K.K., MSD K.K., Ono Pharmaceutical Co., Ltd. and Bristol-Myers Squibb Co. Ltd. outside the submitted work. N.H. reports that this study is supported by Renascience Inc. (to Hiroshima University Hospital). The other authors have no conflicts of interest to declare.
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin 2016;66:7-30. 10.3322/caac.21332 [DOI] [PubMed] [Google Scholar]
- 2.Network NCC. NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®) Non-Small Cell Lung Cancer Version 3. 2023. 2023;April 13, 2023.
- 3.Rodríguez-Abreu D, Powell SF, Hochmair MJ, et al. Pemetrexed plus platinum with or without pembrolizumab in patients with previously untreated metastatic nonsquamous NSCLC: protocol-specified final analysis from KEYNOTE-189. Ann Oncol 2021;32:881-95. 10.1016/j.annonc.2021.04.008 [DOI] [PubMed] [Google Scholar]
- 4.Reck M, Ciuleanu TE, Cobo M, et al. First-line nivolumab plus ipilimumab with two cycles of chemotherapy versus chemotherapy alone (four cycles) in advanced non-small-cell lung cancer: CheckMate 9LA 2-year update. ESMO Open 2021;6:100273. 10.1016/j.esmoop.2021.100273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Johnson ML, Cho BC, Luft A, et al. Durvalumab With or Without Tremelimumab in Combination With Chemotherapy as First-Line Therapy for Metastatic Non-Small-Cell Lung Cancer: The Phase III POSEIDON Study. J Clin Oncol 2023;41:1213-27. 10.1200/JCO.22.00975 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Garon EB, Ciuleanu TE, Arrieta O, et al. Ramucirumab plus docetaxel versus placebo plus docetaxel for second-line treatment of stage IV non-small-cell lung cancer after disease progression on platinum-based therapy (REVEL): a multicentre, double-blind, randomised phase 3 trial. Lancet 2014;384:665-73. 10.1016/S0140-6736(14)60845-X [DOI] [PubMed] [Google Scholar]
- 7.Sun JM, Lee KW, Kim JH, et al. Efficacy and toxicity of pemetrexed as a third-line treatment for non-small cell lung cancer. Jpn J Clin Oncol 2009;39:27-32. 10.1093/jjco/hyn118 [DOI] [PubMed] [Google Scholar]
- 8.Miyoshi S, Ito R, Katayama H, et al. Phase II trial of S-1 as third-line or further chemotherapy in patients with advanced non-small-cell lung cancer. Int J Clin Oncol 2014;19:1005-10. 10.1007/s10147-014-0663-9 [DOI] [PubMed] [Google Scholar]
- 9.Tanaka H, Taima K, Morimoto T, et al. A single-arm phase II study of nab-paclitaxel for patients with chemorefractory non-small cell lung cancer. BMC Cancer 2017;17:683. 10.1186/s12885-017-3684-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yoshioka H, Katakami N, Okamoto H, et al. A randomized, open-label, phase III trial comparing amrubicin versus docetaxel in patients with previously treated non-small-cell lung cancer. Ann Oncol 2017;28:285-91. 10.1093/annonc/mdw621 [DOI] [PubMed] [Google Scholar]
- 11.Kato Y, Okuma Y, Watanabe K, et al. A single-arm phase II trial of weekly nanoparticle albumin-bound paclitaxel (nab-paclitaxel) monotherapy after standard of chemotherapy for previously treated advanced non-small cell lung cancer. Cancer Chemother Pharmacol 2019;84:351-8. 10.1007/s00280-019-03843-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cortot AB, Audigier-Valette C, Molinier O, et al. Weekly paclitaxel plus bevacizumab versus docetaxel as second- or third-line treatment in advanced non-squamous non-small-cell lung cancer: Results of the IFCT-1103 ULTIMATE study. Eur J Cancer 2020;131:27-36. 10.1016/j.ejca.2020.02.022 [DOI] [PubMed] [Google Scholar]
- 13.Planchard D, Reinmuth N, Orlov S, et al. ARCTIC: durvalumab with or without tremelimumab as third-line or later treatment of metastatic non-small-cell lung cancer. Ann Oncol 2020;31:609-18. 10.1016/j.annonc.2020.02.006 [DOI] [PubMed] [Google Scholar]
- 14.Brahmer J, Reckamp KL, Baas P, et al. Nivolumab versus Docetaxel in Advanced Squamous-Cell Non-Small-Cell Lung Cancer. N Engl J Med 2015;373:123-35. 10.1056/NEJMoa1504627 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Herbst RS, Baas P, Kim DW, et al. Pembrolizumab versus docetaxel for previously treated, PD-L1-positive, advanced non-small-cell lung cancer (KEYNOTE-010): a randomised controlled trial. Lancet 2016;387:1540-50. 10.1016/S0140-6736(15)01281-7 [DOI] [PubMed] [Google Scholar]
- 16.Akamatsu H, Teraoka S, Takamori S, et al. Nivolumab Retreatment in Non-Small Cell Lung Cancer Patients Who Responded to Prior Immune Checkpoint Inhibitors and Had ICI-Free Intervals (WJOG9616L). Clin Cancer Res 2022;28:OF1-OF7. 10.1158/1078-0432.CCR-22-0602 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Iwaki T, Urano T, Umemura K. PAI-1, progress in understanding the clinical problem and its aetiology. Br J Haematol 2012;157:291-8. 10.1111/j.1365-2141.2012.09074.x [DOI] [PubMed] [Google Scholar]
- 18.Li S, Wei X, He J, et al. Plasminogen activator inhibitor-1 in cancer research. Biomed Pharmacother 2018;105:83-94. 10.1016/j.biopha.2018.05.119 [DOI] [PubMed] [Google Scholar]
- 19.Masuda T, Hattori N, Senoo T, et al. SK-216, an inhibitor of plasminogen activator inhibitor-1, limits tumor progression and angiogenesis. Mol Cancer Ther 2013;12:2378-88. 10.1158/1535-7163.MCT-13-0041 [DOI] [PubMed] [Google Scholar]
- 20.Takayama Y, Hattori N, Hamada H, et al. Inhibition of PAI-1 Limits Tumor Angiogenesis Regardless of Angiogenic Stimuli in Malignant Pleural Mesothelioma. Cancer Res 2016;76:3285-94. 10.1158/0008-5472.CAN-15-1796 [DOI] [PubMed] [Google Scholar]
- 21.Masuda T, Nakashima T, Namba M, et al. Inhibition of PAI-1 limits chemotherapy resistance in lung cancer through suppressing myofibroblast characteristics of cancer-associated fibroblasts. J Cell Mol Med 2019;23:2984-94. 10.1111/jcmm.14205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Izuhara Y, Takahashi S, Nangaku M, et al. Inhibition of plasminogen activator inhibitor-1: its mechanism and effectiveness on coagulation and fibrosis. Arterioscler Thromb Vasc Biol 2008;28:672-7. 10.1161/ATVBAHA.107.157479 [DOI] [PubMed] [Google Scholar]
- 23.Izuhara Y, Yamaoka N, Kodama H, et al. A novel inhibitor of plasminogen activator inhibitor-1 provides antithrombotic benefits devoid of bleeding effect in nonhuman primates. J Cereb Blood Flow Metab 2010;30:904-12. 10.1038/jcbfm.2009.272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Takahashi N, Kameoka Y, Onizuka M, et al. Deep molecular response in patients with chronic phase chronic myeloid leukemia treated with the plasminogen activator inhibitor-1 inhibitor TM5614 combined with a tyrosine kinase inhibitor. Cancer Med 2023;12:4250-8. 10.1002/cam4.5292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer 2009;45:228-47. 10.1016/j.ejca.2008.10.026 [DOI] [PubMed] [Google Scholar]
- 26.National Cancer Institute. Common Terminology Criteria for Adverse Events (CTCAE) v5.0. Available online: https://ctep.cancer.gov/protocolDevelopment/electronic_applications/ctc.htm#ctc_50
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The article’s supplementary files as