Figure 1: Nucleotide-binding and oligomerizing sensors as a universal strategy for cellular defense.
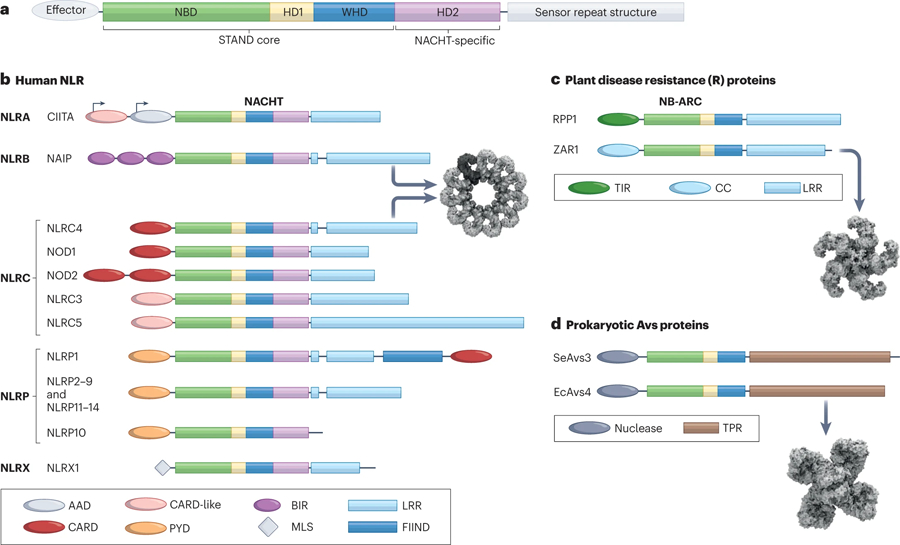
(A) The STAND ATPase module consisting of NBD, helical domain 1 (HD1), and winged helix domain (WHD) is utilized for cellular defense from prokaryotes to eukaryotes. An additional helical domain 2 (HD2) is present in many STAND proteins including a NACHT-specific domain used in the NLR family of proteins. (B) The primary structural organization of human NLRs. Here, NLRs are grouped according to subfamily which is determined by the effector domain at the N-terminus. The variable domains associated with the NACHT domain are color coded and indicated in the domain legend below. CIITA exhibits cell-type specific alternative promoter usage, and this is designated with arrows. The oligomerized inflammasome for NAIP/NLRC4 is shown (PDB:3JBL)288. (C) Two representative members of the plant disease resistance proteins with either TIR or CC domains are shown. The NB-ARC module lacks the HD2 domain. The C-terminal sensor is an LRR domain for these proteins as well. The oligomerized resistosome for ZAR1 is shown (PDB:6J5T)327. (D) Two representative members of the recently described prokaryotic Avs protein family are shown. The effector domain in these proteins is a nuclease instead of a protein-recruitment domain, and the sensor domain is composed of tetratricopeptide repeats (TPR). The oligomerized complex for EcAvs4 is shown (PDB:8DGF)27. Cryo-EM structure representations in this figure were created with BioRender.com.
