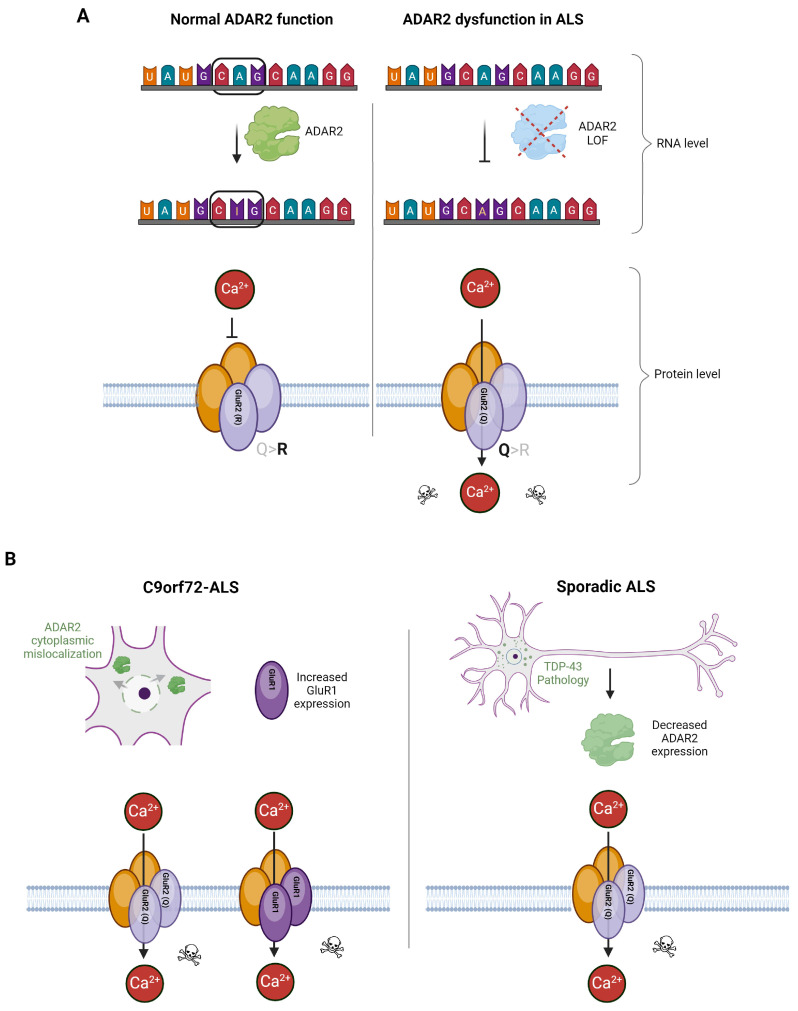
Figure 2.
GluR2 editing by the ADAR2 enzyme and AMPAR composition in ALS. (A) The ADAR2 enzyme converts the GluR2 mRNA CAG codon to a CIG codon at the RNA level (left panel). This A-to-I RNA editing changes the gene-encoded glutamine to a positively charged arginine residue at the protein level. The incorporation of ADAR2-edited GluR2 results in a calcium-impermeable AMPAR (left panel). This RNA editing dysfunction allows calcium permeability of the AMPAR (right panel) and downstream excitotoxicity [10]. (B) In both FALS and SALS, multiple mechanisms have been proposed that ultimately result in increased calcium permeability of AMPARs. In C9orf72-ALS (left panel), nucleocytoplasmic mislocalization of ADAR2 has been observed in multiple model systems as well as in postmortem patient spinal cord tissue. As described, this leads to reduced editing of the GluR2 mRNA and an increase in AMPAR calcium permeability [44]. Additionally, increased expression of calcium-permeable GluR1 was reported in C9orf72-ALS iPSC-derived motor neurons. In SALS (right panel), TDP-43 pathology corresponds with decreased ADAR2 expression, which also results in decreased editing of the GluR2 mRNA and increased calcium permeability via AMPARs.