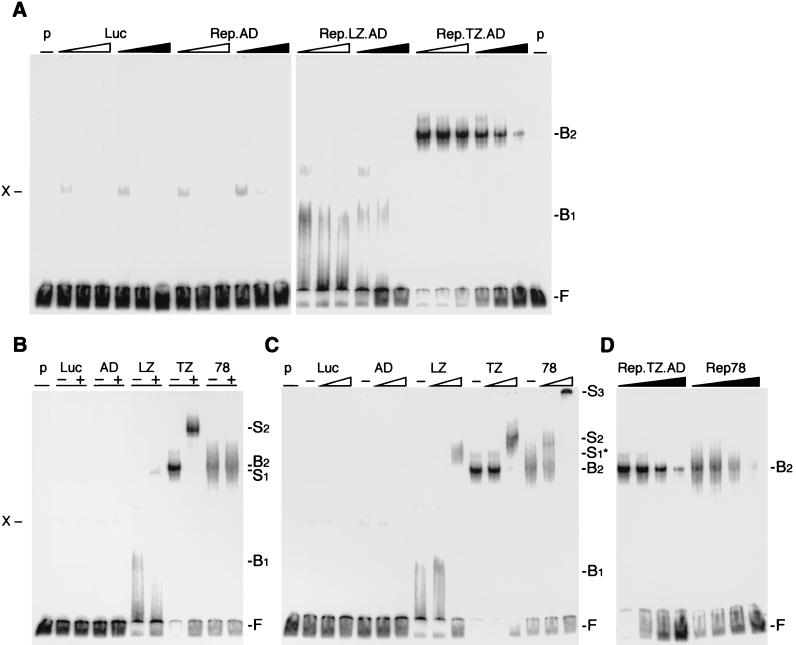
FIG. 3.
EMSAs of Rep fusion proteins. Chimeric Rep proteins were synthesized in vitro in a rabbit reticulocyte lysate and incubated with the 3′-end-labeled RRS probe. In vitro-translated luciferase (Luc) served as a negative control for the lysate, and nonspecific bands are indicated (X). The positions of free (F), bound (B), and supershifted (S) DNA substrate are indicated on the right. (See text for an explanation of the bound and supershifted complexes.) (A) The chimeric Rep proteins bind to the RRS in a specific manner. Increasing molar ratios (5×, 25×, and 125×) of unlabeled DNA fragments containing the RRS (black triangles) or a mutant RRS (open triangles) were added to the reaction as competitors. (B and C) The DNA-bound, chimeric Rep proteins are supershifted with specific antibodies to the Myc tag (B) or Rep (C). The absence (−) or presence (+) of the antibody is indicated on top. Triangles indicate increasing amounts (1/30 and 1 μl) of the polyclonal anti-Rep antibody. (D) Rep.TZ.AD binds to the RRS with similar affinity as Rep78. Increasing molar ratios of unlabeled RRS probe (0, 5×, 25×, 125×) were added as competitor to the reaction. p, probe; Luc, luciferase; AD, Rep.AD; LZ, Rep.LZ.AD; TZ, Rep.TZ.AD; 78, Rep78.