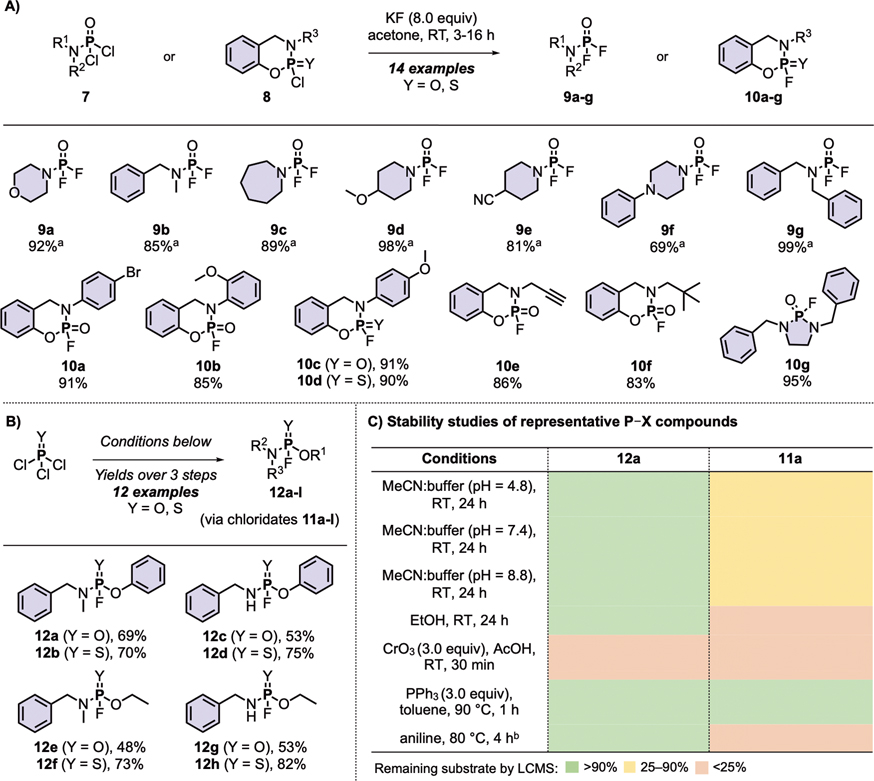
Scheme 1. Synthesis of PFEx substrates.
(A) Synthesis of phosphoramidic difluorides, cyclic phosphoramidofluoridates, and cyclic thiophosphoramidofluoridates.
(B) Synthesis of phosphoramidofluoridates and thiophosphoramidofluoridates. General reaction conditions: POCl3 or PSCl3 (1.0 equiv) and Et3N (1.0 equiv) were added to relevant phenol (1.0 equiv) in CH2Cl2 (0.25 M) at −78 °C then stirred overnight at room temperature. The required amine (1.0 equiv) was added, followed by Et3N (1.0 equiv) dropwise at −78 °C. The reaction was stirred at room temperature until complete (31P NMR). The reaction was filtered and concentrated. KF (8.0 equiv) and nBu4NCl (0.10 equiv) were added to the crude in acetone (0.25 M). After completion (31P NMR), the reaction was filtered, concentrated, and purified by silica column chromatography. Isolated yields are reported. Reactions were performed on 5.0 mmol following general procedures detailed in the supporting information unless stated otherwise. See supporting information for a complete list of products.
(C) Stability studies of representative P–X compounds.
[a]Product had limited stability.
[b]11a was completely consumed after 1 h; P–Cl exchange product was identified.