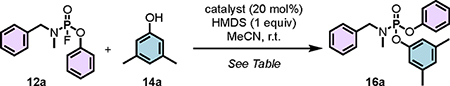
Table 1.
PFEx catalyst screen.
| ||||
|---|---|---|---|---|
|
| ||||
| Entry | Catalyst | pKaH (MeCN) | Time (h) | Conversion (%)a |
|
| ||||
| 1 | P4-tBu | 42.7 | 1 | >99 |
| 2 | P2-tBu | 33.5 | 1.5 | >99 |
| 3 | TBD | 26.2 | 5 | >99 |
| 4 | BTMG | ~26 | 14 | 91 |
| 5 | DBU | 24.3 | 14 | 80 |
| 6 | P1-tBu | 26.9 | 14 | 10 |
| 7 | TMG | 23.7 | 14 | 9 |
| 8 | DPG | 18.8 | 14 | Trace |
| 9 | BEMP | 27.5 | 14 | 66 |
| 10 | MTBD | 25.0 | 14 | 64 |
| 11 | DMAP | 18.0 | 14 | 0 |
| 12 b | TBD | 26.2 | 2 | >99 |
| 13c | TBD | 26.2 | 7 | 16 |
| 14d | TBD | 26.2 | 7 | 20 |
Reactions were conducted on a 0.10 mmol scale in acetonitrile (0.25 M). Refer to supplementary information Figure S4 for a list of catalyst structures.
Conversions were determined by 31P NMR and 19F NMR.
1.20 equiv HMDS and 3, 5-dimethyphenol in MeCN (0.5 M) were employed.
HMDS was replaced with Et3N (1.0 equiv).
Without HMDS.
