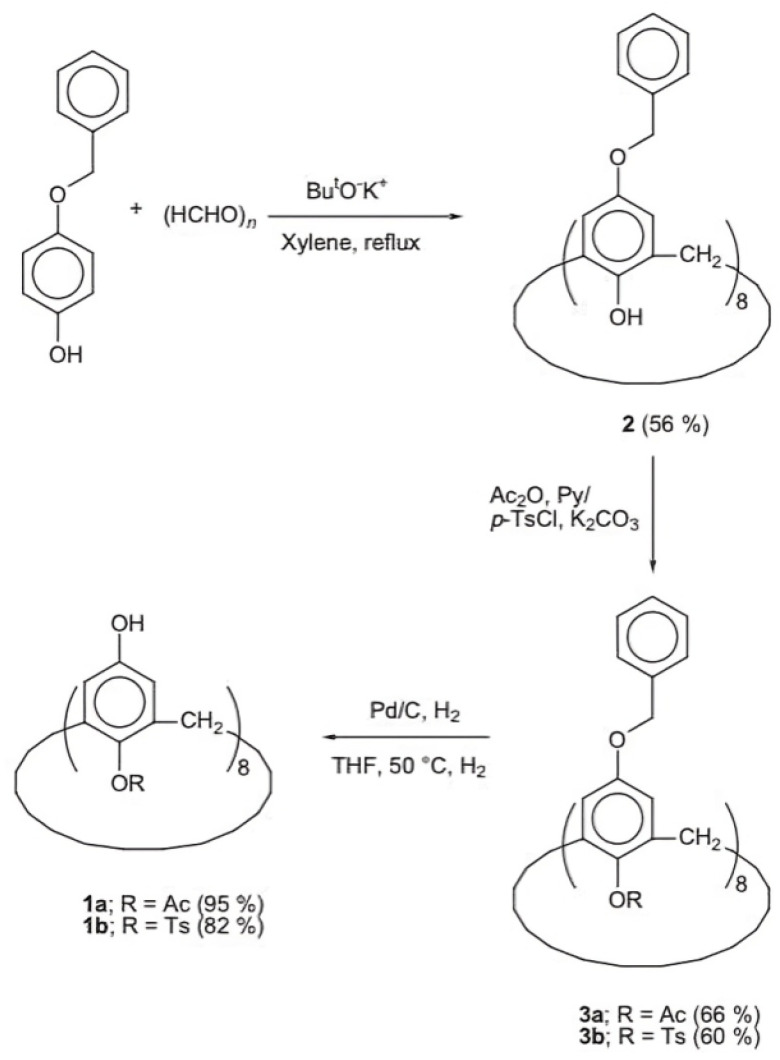
Figure 3.
The synthesis scheme illustrates the stepwise process for generating O-substituted hydroxyphenol calix[8]arenes: 1a: The preparation of p-(mono-acetyl)-calix[8]arene with a yield of 95%. 1b: The preparation of p-(p-tolylsulfonyl)-calix[8]arene with a yield of 82%. 2: The synthesis of p-(benzyloxy)-calix[8]arene. 3a: Acetylation of Compound 2, resulting in p-(mono-acetyl)-calix[8]arene with a yield of 66%. 3b: Reaction of Compound 2 with toluene-p-sulfonyl chloride, yielding p-(p-tolylsulfonyl)-calix[8]arene with a yield of 60% [25].