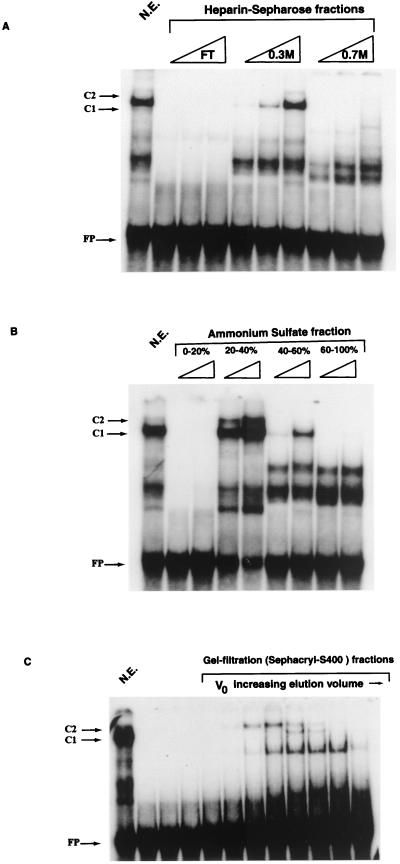
FIG. 6.
Biochemical enrichment of a principal factor binding numerous sites in the 5′LCR-MAR and the E6-MAR. The purification profile of the factor binding E6-10, as monitored by EMSA, is similar to that of the differentiation-specific factor CDP/Cut. (A) Ion-exchange chromatography purification of HeLa nuclear extracts (N.E.) on a heparin-Sepharose column demonstrates that PSM binding protein activity is maximal in the 0.3 M KCl fraction. FT, flowthrough. (B) Ammonium sulfate precipitation of E6-10 binding fractions of the heparin-Sepharose chromatography defines the activity in the 20 to 40% fraction. (C) A gel filtration experiment, with the 20 to 40% ammonium sulfate fraction from the heparin-Sepharose column, indicates that the protein binding E6-10 is a very large protein of approximately 180 kDa (as defined by the β-amylase molecular weight marker). V0, void volume; FP, free probe. The purification profile presented here is consistent with that described for the differentiation-specific transcriptional repressor CDP/Cut (44).