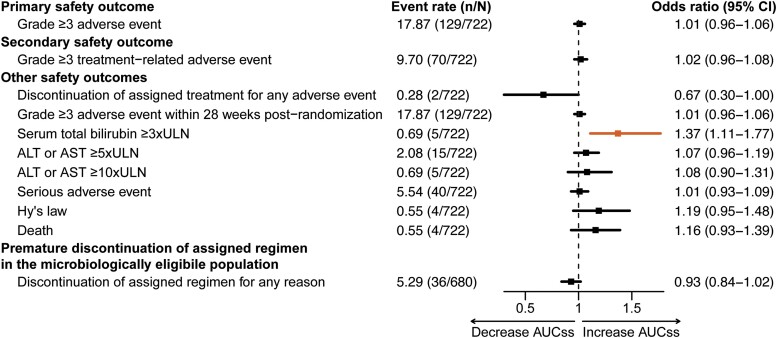
Figure 6.
Odds of safety outcomes by rifampicin exposure. Odds ratios were calculated per 5-mg·h/L increase in rifampicin AUCss after adjustment for pyrazinamide AUCss, age, and body weight. The safety outcome is highlighted in orange if it was found to be statistically significant with a P value <.05. Safety and tolerability outcomes were defined as those occurring during treatment and up to 14 days after discontinuation of study drug. Abbreviations: ALT, alanine aminotransferase; AST, aspartate aminotransferase; AUCss, area under the plasma concentration-time curve at steady state; CI, confidence interval; n/N, number of participants reported for each safety outcome over of the total number of participants analyzed; ULN, upper limit of normal.