Abstract
Background
Asymptomatic SARS-CoV-2 infection in children is highly prevalent but its acute and chronic implications have been minimally described.
Methods
In this controlled case-ascertained household transmission study, we recruited asymptomatic children <18 years with SARS-CoV-2 nucleic acid testing performed at 12 tertiary care pediatric institutions in Canada and the United States. We attempted to recruit all test-positive children and 1 to 3 test-negative, site-matched controls. After 14 days’ follow-up we assessed the clinical (ie, symptomatic) and combined (ie, test-positive, or symptomatic) secondary attack rates (SARs) among household contacts. Additionally, post–COVID-19 condition (PCC) was assessed in SARS-CoV-2–positive participating children after 90 days’ follow-up.
Results
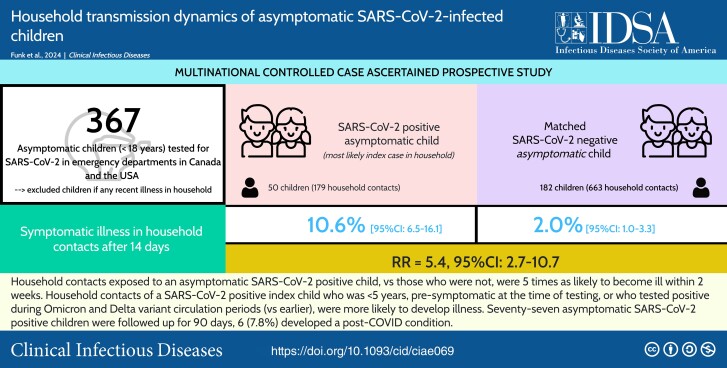
A total of 111 test-positive and 256 SARS-CoV-2 test-negative asymptomatic children were enrolled between January 2021 and April 2022. After 14 days, excluding households with co-primary cases, the clinical SAR among household contacts of SARS-CoV-2–positive and –negative index children was 10.6% (19/179; 95% CI: 6.5%–16.1%) and 2.0% (13/663; 95% CI: 1.0%–3.3%), respectively (relative risk = 5.4; 95% CI: 2.7–10.7). In households with a SARS-CoV-2–positive index child, age <5 years, being pre-symptomatic (ie, developed symptoms after test), and testing positive during Omicron and Delta circulation periods (vs earlier) were associated with increased clinical and combined SARs among household contacts. Among 77 asymptomatic SARS-CoV-2–infected children with 90-day follow-up, 6 (7.8%; 95% CI: 2.9%–16.2%) reported PCC.
Conclusions
Asymptomatic SARS-CoV-2–infected children, especially those <5 years, are important contributors to household transmission, with 1 in 10 exposed household contacts developing symptomatic illness within 14 days. Asymptomatic SARS-CoV-2–infected children may develop PCC.
Keywords: asymptomatic SARS-CoV-2, children, household transmission, prospective cohort, post-COVID-19 condition
Household contacts of SARS-CoV-2 test-positive asymptomatic children were 5 times more likely to develop symptomatic illness within 2 weeks compared with household contacts of test-negative asymptomatic children. Post-COVID condition was reported by 8% of initially asymptomatic SARS-CoV-2–infected children.
Graphical Abstract
Graphical Abstract.
This graphical abstract is also available at Tidbit: https://tidbitapp.io/tidbits/household-transmission-dynamics-of-asymptomatic-sars-cov-2-infected-children-a-multinational-controlled-case-ascertained-prospective-study
The role of children in severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) household transmission has been poorly quantified. Early meta-analyses reported that children were infrequently the index case in household clusters, with limited transmission to household contacts [1]. While recent studies have identified symptomatic children as important contributors to household transmission [2–6], studies of asymptomatic pediatric index cases have included few participants or have not presented results of this population separately [4, 5, 7–10].
Approximately one-third of all documented SARS-CoV-2 infections are asymptomatic [11–13], and the true proportion of asymptomatic infections is likely higher [14, 15]. Moreover, although the risk of developing post–coronavirus disease 2019 (COVID-19) condition (PCC) or “long COVID” in asymptomatically infected adults is estimated to be 20% [16], the natural history of asymptomatic SARS-CoV-2 infection in children is poorly characterized. Only a handful of studies have reported on PCC in children with asymptomatic SARS-CoV-2 infection, with risk estimates ranging from 0% to 27% [17–20]. Until these risks are better clarified, asymptomatic infections, which are common with the Omicron variant, cannot be considered benign [21].
The SARS-CoV-2 virus is expected to become an endemic infectious disease in humans [22]. Although the COVID-19 pandemic is no longer considered a public health emergency of international concern, pediatric COVID-19 hospitalizations and coinfections in the United States are increasing [23, 24]. Our knowledge of how COVID-19 pandemic control measures have impacted children will surely influence future policy. However, these decisions need to also be informed by the acute and chronic impacts of pediatric SARS-CoV-2 infection. To fill this knowledge gap, we aimed to quantify the risk of transmission from infected asymptomatic children to their household contacts within 14 days of testing positive for SARS-CoV-2 and to identify associated risk factors. We also sought to estimate the risk of PCC in asymptomatic, acutely infected children.
METHODS
Study Design and Recruitment
We conducted a controlled, case-ascertained, 14-day household transmission study of asymptomatic SARS-CoV-2–infected and noninfected children. We also prospectively followed SARS-CoV-2–infected participants for 90 days. Children under 18 years of age without COVID-19 symptoms (ie, fever, cough, difficulty breathing, fatigue/weakness, myalgias, chills, feeling very unwell, sore throat, rhinorrhea, vomiting, diarrhea, conjunctivitis, headache, anosmia, ageusia, rash) within the preceding 24 hours who were tested for SARS-CoV-2 infection because of a known positive non-household contact, or as part of standard hospital screening procedures, were eligible if they had at least 1 household contact. Household contact was defined as anyone who slept at least 1 night in the household from 2 weeks prior and until 2 weeks after the day of the participating child's SARS-CoV-2 test. Children tested for SARS-CoV-2 via a nucleic acid amplification test (NAAT) were identified and recruited from emergency departments or other hospital-based outpatient/ambulatory settings at 12 pediatric tertiary hospitals in Canada and the United States. For each enrolled SARS-CoV-2–positive child, research personnel attempted to enroll 1–3 asymptomatic children at the same site who tested negative for SARS-CoV-2. After test results were received (within 5 days of testing), a research assistant contacted the legal guardians of potentially eligible children to confirm eligibility; all participants provided written or verbal informed consent and/or assent prior to study activities. Protocols and procedures were approved by the institutional review boards at all study sites.
Data Collection
Baseline data, including demographic characteristics, past medical history, reason for the initial hospital visit, and risk factors for SARS-CoV-2 infection within the previous 14 days, were collected in person, via telephone, or electronic survey, as per participant preference. Fourteen days following SARS-CoV-2 testing, participants were asked for the number of persons in the household and their relationship to the participating child. At that time, all household contacts were asked about symptoms experienced (with onset dates) and the results of any SARS-CoV-2 tests in the 14-day time periods prior to and following the participating child's test, as well as prior known SARS-CoV-2 infection and vaccination status. Data related to the initial hospital visit and any visits occurring in the subsequent 14 days were extracted from participant’s medical records. Ninety days following the initial SARS-CoV-2 test, we administered a final questionnaire for SARS-CoV-2–positive participants regarding PCC.
Viral Load Quantification and Variant Identification
Participating sites were asked to send SARS-CoV-2–positive nasopharyngeal swabs to ProvLab Alberta (University of Alberta, Edmonton, Canada) for viral load quantification and COVID-19 variant identification [25]. Further details are provided in Supplementary Text 1. Viral load was reported as genome copies/mL of sample and, for descriptive analysis, they were categorized as less than 4, 4 to less than 6, and 6 or more log10 copies/mL.
Definitions
Hereafter, we refer to households as either exposed or unexposed, depending on the presence/absence of a SARS-CoV-2–positive pediatric participant at the time of enrollment. SARS-CoV-2–positive children were considered the household index case if no other household contacts had COVID-19 symptoms or a positive SARS-CoV-2 test in the 14 days prior to, and until 24 hours after (ie, co-primary case), their test. Households not fulfilling these criteria (ie, contact with symptoms or positive test within the mentioned time period) were excluded from the primary analysis. For comparison purposes, when the participating child was SARS-CoV-2 negative, the same criteria were applied to identify unexposed households without other known active cases of infectious illness at the time of enrollment. Participants were considered pre-symptomatic if they developed symptoms during the 14-day follow-up. We categorized participants by typical school-age groupings: younger than 5 years, 5 to younger than 13 years, and 13 to younger than 18 years. Household contact age was categorized as younger than 18 years, 18 to younger than 50 years, and 50 years or older. We categorized the length of hospitalization as follows: none (ie, discharged within 24 hours), 24 hours or longer, or unknown. SARS-CoV-2 vaccination status was classified as none or 1 or more dose (ie, any vaccination received). Based on publicly available Canadian data [26], which were analogous to US data [27], participants enrolled from 31 January 2021 (enrollment start) until 30 June 2021, 1 July 2021 to 19 December 2021, and from 21 December 2021 to 22 April 2022 (enrollment end) were classified as being infected during periods of mixed (ie Alpha/other), Delta, and Omicron variant-of-concern (VoC) circulation, respectively.
Participating children were considered lost to follow-up if 3 consecutive attempts to reach out to legal guardians were unsuccessful; 90-day follow-up was attempted regardless of 14-day loss-to-follow-up. As per the World Health Organization consensus definition for children and adolescents, we defined PCC for asymptomatic SARS-CoV-2–infected children as symptoms persistent for at least 2 months with onset occurring within 3 months of confirmed infection [28]. We also considered children to have PCC if they reported a newly diagnosed syndrome or chronic condition since they were tested for SARS-CoV-2 [19].
Outcomes
Primary
The clinical secondary attack rate (SAR) was the proportion of household contacts who became clinical secondary cases (ie, developed symptoms) during the 14-day follow-up period. As has been done with influenza [29, 30], we assumed that, if an acute illness developed in a household contact more than 24 hours after the index child tested positive for SARS-CoV-2, the household contact was infected with SARS-CoV-2 as a “secondary case,” even though directionality cannot be assigned with certainty. The combined SAR consisted of clinical secondary cases plus asymptomatic household contacts who reported a positive SARS-CoV-2 test during follow-up at least 24 hours after the child's SAR-CoV-2 test. The clinical SAR, rather than the combined SAR, was selected for the primary comparisons of transmission in exposed and unexposed households as SARS-CoV-2 testing of household contacts in exposed households was more likely to occur and thus bias our findings. On the other hand, the combined SAR was used for all unadjusted and adjusted analyses that were restricted to exposed households only.
Secondary
The secondary outcome was PCC after 90 days of follow-up.
Statistical Analysis
The characteristics of SARS-CoV-2–positive and –negative participants, and those who completed follow-up versus those lost to follow-up, were compared using chi-square tests or Fisher's exact tests, as appropriate. Viral loads between pre-symptomatic and asymptomatic SARS-CoV-2–positive children were compared using an exact 2-sample Wilcoxon rank-sum test. The proportion of SARS-CoV-2–positive children who developed PCC was estimated along with 95% confidence intervals (CIs).
Unadjusted relative risks (RRs) were calculated to compare household clinical and combined SARs based on the participating child's SARS-CoV-2 status and the household contact's age category. Unadjusted subgroup analysis further generated RRs for household transmission among exposed households after stratifying by a priori specified factors—index child's age, symptom status after 14 days’ follow-up (ie, asymptomatic vs pre-symptomatic), hospitalization length, and vaccination status. For exposed households, a log Poisson generalized estimating equation (GEE) model with robust standard errors [31], accounting for household clustering, was built to generate adjusted incidence rate ratios of risk factors for household contacts becoming secondary cases during the 14-day follow-up. This model was first fitted for all household contacts, with backwards selection of variables based on significance within, and influence on, model estimates. To further examine the influence of the child's vaccination status on transmission, the final model variable selection was applied to the subset of households for whom the index SARS-CoV-2–positive child was at least 5 years old, as this was the lower age limit for vaccination during the study period.
All statistical tests were 2-sided, and P values < .05 were considered significant. Stata IC 16.1 (StataCorp LLC, College Station, TX, USA) was used for all analysis and graphics.
RESULTS
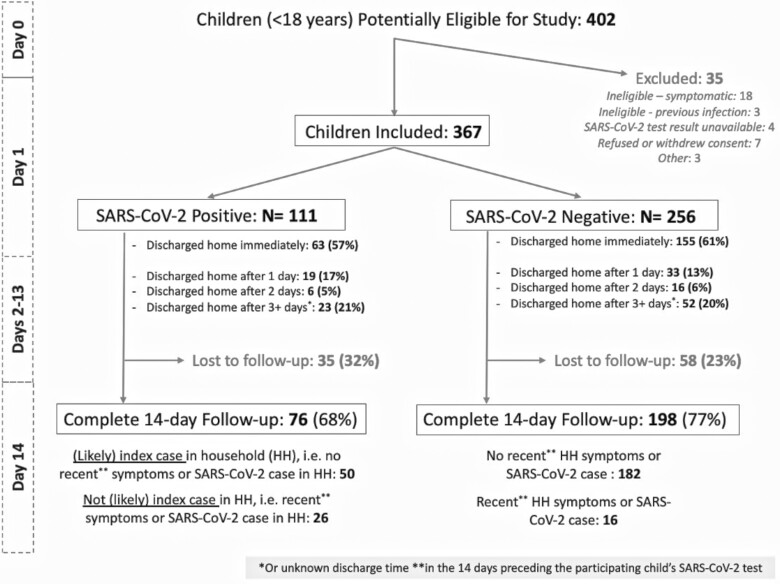
We included 111 asymptomatic SARS-CoV-2 test-positive and 256 asymptomatic test-negative children; 14-day follow-up was completed for 68.5% (n = 76) and 77.3% (n = 198) of participants in these groups, respectively (Figure 1). SARS-CoV-2–positive and –negative children had similar baseline characteristics, except that test-positive children were more likely to report a SARS-CoV-2–positive close contact in the previous 14 days (Table 1, Supplementary Table 1a and 1b). Children lost to follow-up were more likely to be younger than 5 years of age (Supplementary Table 2). We observed that some risk factors for acquiring SARS-CoV-2, including school and daycare attendance and public transit use, increased in later pandemic stages (Supplementary Table 3).
Figure 1.
Follow-up of pediatric asymptomatic SARS-CoV-2–positive and –negative participants until 14 days after testing. Abbreviation: SARS-CoV-2, severe acute respiratory syndrome coronavirus 2.
Table 1.
Baseline Characteristics of Asymptomatic Participating Children Included in Transmission Analysis (ie, Complete 14-Day Follow-up)
| SARS-CoV-2 Status | All Participants | ||
|---|---|---|---|
| Positive (n = 76) | Negative (n = 198) | P | |
| Demographic and clinical characteristics | |||
| Male | 45 (59.2) | 112 (56.6) | .69 |
| Country | .39 | ||
| Canada | 19 (25.0) | 40 (20.2) | |
| United States | 57 (75.0) | 158 (79.8) | |
| Chronic illness (excluding asthma)a | 18 (23.7) | 35 (17.7) | .26 |
| Psycho-behavioral | 7 (9.2) | 7 (3.5) | .21 |
| Neurodevelopmental | 6 (7.9) | 12 (6.1) | .58 |
| Pulmonary | 2 (2.6) | 2 (1.0) | .31 |
| Cardiac | 1 (1.3) | 3 (1.5) | 1.00 |
| Kidney or liver | 1 (1.3) | 4 (2.0) | 1.00 |
| Hematologic | 0 | 6 (3.0) | .19 |
| Diabetes | 0 | 2 (1.0) | 1.00 |
| Other | 2 (2.6) | 12 (6.1) | .36 |
| Asthma | 7 (9.2) | 15 (7.6) | .66 |
| Hospital length of stay (this visit) | .85 | ||
| Discharged home immediately | 47 (61.8) | 124 (62.6) | |
| 1 day | 11 (14.5) | 26 (13.1) | |
| 2 days | 4 (5.3) | 14 (7.1) | |
| 3+ days | 8 (10.5) | 14 (7.1) | |
| Unknown | 6 (7.9) | 20 (10.1) | |
| Number of household contactsb (median, IQR) | 4 (4–5.5) | 4 (4–5) | .67 |
| Transmission risk or protective factors in the 14 days prior to SARS-CoV-2 test | |||
| Had SARS-CoV-2–positive close contact | 36 (47.4) | 26 (13.1) | <.001 |
| Attended daycare or school | 41 (54.0) | 110 (55.6) | .81 |
| Attended a social gatheringa | |||
| 10 to <50 persons | 15 (19.7) | 31 (15.7) | .42 |
| >50 persons | 5 (6.6) | 18 (9.1) | .63 |
| Used public transitc,d | 9 (11.8) | 38 (19.2) | .15 |
| Wore a mask in publicc | 59 (77.6) | 145 (73.2) | .46 |
| Washed or disinfected handsc,e | 66 (86.8) | 159 (80.3) | .21 |
| SARS-CoV-2 vaccine, ≥1 dose | 20 (26.3) | 46 (23.2) | .59 |
Data are n (%) unless otherwise stated.
Abbreviations: IQR, interquartile range; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2.
aSuboptions not mutually exclusive.
bIncludes participant.
c“Sometimes” or “Always”.
dIncludes bus, train, taxi, and rideshare.
eAsked if children disinfected/washed hands upon home arrival.
Secondary Attack Rate
After 14 days, for all 76 households (275 household contacts) who completed follow-up of an asymptomatic SARS-CoV-2–positive child, the combined and clinical SARs were 19.6% (95% CI: 15.1%–24.8%) and 13.5% (95% CI: 9.7%–18.1%), respectively. Among the 50 exposed households where the asymptomatic SARS-CoV-2–positive child was the index case, with 179 household contacts, the combined and clinical SARs were 14.0% (95% CI: 9.2%–19.9%) and 10.6% (95% CI: 6.5%–16.1%), respectively. Among unexposed households (ie, SARS-CoV-2–negative child) with no symptomatic household contacts at baseline, the clinical SAR was 2.0% (95% CI: 1.0%–3.3%; RR exposed vs unexposed, 5.4; 95% CI: 2.7–10.7). The clinical SAR RR between exposed and unexposed households differed by household contact age group, being greatest for household contacts younger than 18 years (RR, 11.9; 95% CI: 1.3–112.5) (Table 2, Supplementary Table 4). A stratified analysis demonstrated that, when the index child had a known SARS-CoV-2–positive non-household exposure, the clinical SARs were similar for SARS-CoV-2–positive exposed and unexposed households (RR = 1.4; 95% CI: .5–3.7), whereas when there was no known external exposure, the clinical SARs differed significantly (RR = 8.1; 95% CI: 3.2–20.5) (Supplementary Table 5).
Table 2.
Symptoms, Secondary Attack Rates, and Relative Risks for Contacts in Households Without Co-Primary Cases
| Index Child's SARS-CoV-2 Status | All | HH Contact Age Group | ||||||
|---|---|---|---|---|---|---|---|---|
| <18 Years | 18 to <50 Years | 50+ Years | ||||||
| Positivea | Negativeb | Positive | Negative | Positive | Negative | Positive | Negative | |
| No. (% within SARS-CoV-2 status group) | 179 (50 HH) | 663 (182 HH) | 62 (34.3) | 246 (37.1) | 92 (51.4) | 346 (52.2) | 22 (12.2) | 63 (9.5) |
| Any symptoms (clinical SAR) [95% CI] | 19 (10.6)[6.5–16.1] | 13 (2.0)[1.0–3.3] | 3 (4.8)[1.0–13.5] | 1 (0.4)[0–13.5] | 15 (16.1)[9.4–25.5] | 10 (2.9)[1.4–5.3] | 1 (4.5)[.1–22.8] | 2 (3.2)[0.4–11.0] |
| RR [95% CI] by HH contact age group (within SARS-CoV-2 status group) | … | … | ref | ref | 3.4 [1.0–11.2] | 7.1 [.9–55.2] | 0.9 [.1–8.6] | 7.8 [0.7–84.8] |
| RR [95% CI], SARS-CoV-2–positive vs –negative (within HH contact age group) | 5.4 [2.7–10.7] | ref | 11.9 [1.3–112.5] | ref | 5.6 [2.6–12.1] | ref | 1.4 [.1–15.0] | ref |
| Acute respiratory illnessc (%) [95% CI] | 7 (3.9)[1.6–7.9] | 7 (1.1)[.4–2.2] | 1 (1.6)[0–8.7] | 0[0–1.5] | 5 (5.4)[1.8–12.2] | 5 (1.4)[.5–3.3] | 1 (4.5)[.1–22.8] | 2 (3.2)[.4–11.0] |
| RR [95% CI] by HH contact age group (within SARS-CoV-2 status group) | … | … | ref | ref | 3.4 [0.4–28.1] | 7.1 [.9–55.2] | 2.8 [.2–43.2] | 0.5 [0–5.4] |
| RR [95% CI], SARS-CoV-2–positive vs –negative (within HH contact age group) | 3.7 [1.3–10.4] | ref | Excluded | ref | 3.8 [1.1–12.7] | ref | 1.4 [.1–15.0] | ref |
| Any known illnessd (combined SAR) [95% CI] | 25 (14.0)[9.2–19.9] | 13 (2.0)[1.0–3.3] | 6 (9.7) | 1 (0.4)[0–13.5] | 18 (19.6)[12.0–29.1] | 10 (2.9)[1.4–5.3] | 1 (4.5)[.1–22.8] | 2 (3.2)[.4–11.0] |
| RR [95% CI] by HH contact age group (within SARS-CoV-2 status group) | … | … | ref | ref | 2.0 [.9–4.8] | 7.1 [.9–55.2] | 0.5 [.1–3.7] | 7.8 [.7–84.8] |
| RR [95% CI], SARS-CoV-2–positive vs –negative (within HH contact age group) | 7.1 [3.7–13.6] | ref | 23.8 [2.9–194.1] | ref | 6.8 [3.2–14.2] | ref | 1.4 [.1–15.0] | ref |
Data are n (%) unless otherwise stated.
Abbreviations: CI, confidence interval; HH, household; ref, reference; RR, relative risk; SAR, secondary attack rate; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2.
aSpecific age missing for 3 HH contacts.
bSpecific age missing for 8 HH contacts.
cAt least 2 of: fever or feverishness, cough, sore throat, runny nose.
dTest-positive and/or any symptoms.
Risk Factors for Household Transmission
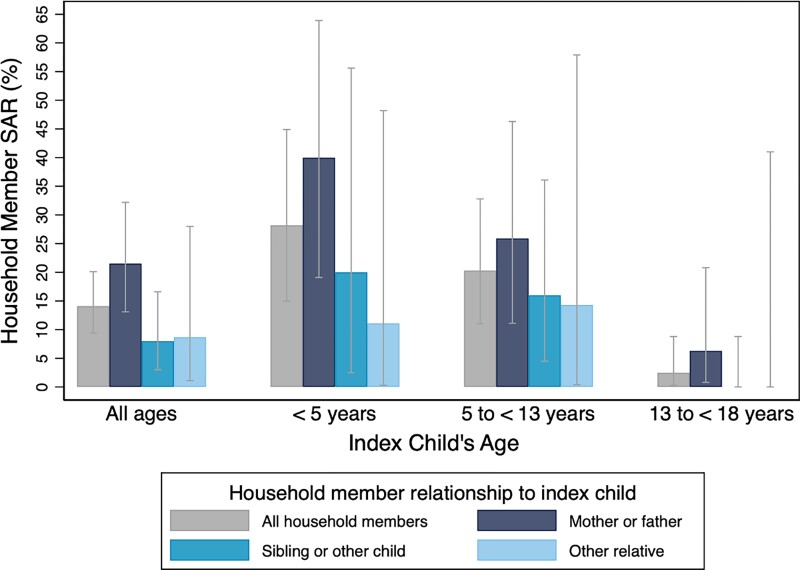
The combined SAR among household contacts of SARS-CoV-2–positive pediatric index cases was higher if the index child was younger than 5 years, or 5 to younger than 13 years, compared to 13 to younger than 18 years of age (RRs = 11.1 [95% CI: 2.6–47.8] and 8.0 [95% CI: 1.9–34.5], respectively) (Figure 2, Supplementary Figures 1 and 2). Unadjusted analysis also demonstrated that the combined SAR was higher among contacts of children discharged compared with those admitted to the hospital (RR = 3.0; 95% CI: 1.1–8.2) (Supplementary Table 6), that caregiver household contacts (ie, mother, father, or equivalent) were more likely to be secondary cases compared with siblings and other children (RR = 2.7; 95% CI: 1.1–6.5), and that pre-symptomatic index cases led to higher combined SARs (42.3%; 95% CI: 23.4%–63.1%) compared with fully asymptomatic index cases (9.2%; 95% CI: 5.1%–14.9%) (Supplementary Table 7). Combined SARs also differed according to whether or not the household contacts had previous known infection or vaccination (Supplementary Table 8). In the adjusted GEE model, index case age younger than 5 years vs 13 to younger than 18 years, being pre-symptomatic vs never symptomatic, and being enrolled when the Delta and Omicron variants were circulating vs Alpha/other were associated with increased combined SARs (Supplementary Tables 9 and 10).
Figure 2.
Household (HH) contact combined SARs (ie, test and/or symptoms after 14 days), according to the index child's age group and HH contact relationship to the index child, for 50 households (177* HH members) with a SARS-CoV-2–positive asymptomatic** index child. Fisher's exact test P values for HH SARs: across index child age groups = <.001; by relationship to index child = 0.043, *2 of the total 179 HH members were missing information on relationship to index child; **asymptomatic at the time of testing. Abbreviations: SAR, secondary attack rate; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2.
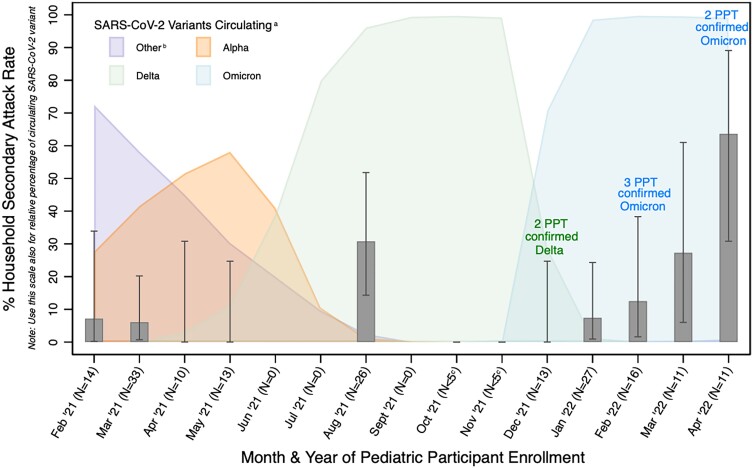
Of 111 participating asymptomatic SARS-CoV-2–positive children, 17 and 11 had viral load and VoC testing performed, respectively, thus limiting comparative analyses (Supplementary Table 11). The combined SAR was higher among Delta and Omicron time-period index cases than among Alpha/other VoC (Figure 3, Supplementary Figure 3).
Figure 3.
Household (HH) contact combined SARs (ie, test and/or symptoms after 14 days), by time of SARS-CoV-2–positive asymptomatic index participant enrollment, with relative percentage of circulating SARS-CoV-2 variants: 179 HH contacts, 50 index participants (ie, HH). Secondary attack rate among household contacts includes either becoming symptomatic or test-positive within 14 days. aUsing publicly available Canadian data (health-infobase.canada.ca/covid.19/testing-variants.html), which are analogous to US CDC data (covid.cdc.gov/covid-data-tracker/#variant-summary). bIncludes gamma, eta, beta, and other variants. cData from months with <5 household contacts (hence, very large confidence intervals) excluded from figure for visual purposes. Abbreviations: CDC, Centers for Disease Control and Prevention; N, number of household contacts for pediatric participants enrolled during that time; PPT, index pediatric participant; SAR, secondary attack rate; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2.
Post–COVID-19 Condition
Among the 77 SARS-CoV-2–positive children who completed 90-day follow-up (Figure 1), 6 (7.8%; 95% CI: 2.9%–16.2%) reported PCC; 5 of these children had persistent symptoms and 1 child had a newly diagnosed syndrome. This included 2 children who were younger than 5 years (7.7%; 95% CI: .9%–25.1%), 1 child aged 5 to younger than 13 years (3.4%; 95% CI, .1%–17.8%), and 3 children aged 13 to younger than 18 years (13.6%; 95% CI: 1.1%–29.2%) (Supplementary Table 12). All 6 children remained asymptomatic during acute infection (ie, the first 14 days of follow-up).
DISCUSSION
In this multicenter, case-ascertained household transmission study, 11% of household contacts, where an asymptomatic SARS-CoV-2–positive child was the index case, developed symptoms consistent with COVID-19 within 14 days, and 15% either developed symptoms or reported a positive SARS-CoV-2 test. Through follow-up of a comparable group of asymptomatic SARS-CoV-2 test-negative children, we determined that the risk of developing symptomatic illness within 14 days was 5 times greater among household contacts of asymptomatic SARS-CoV-2–positive children. The risk of household transmission was greater when the asymptomatic child was younger, pre-symptomatic, and tested positive during periods of Delta and Omicron circulation. After 90 days, approximately 1 in 13 SARS-CoV-2–positive, asymptomatically infected children reported PCC.
Our estimate that 15% of household contacts of an asymptomatic pediatric index case became a secondary case within 14 days is lower than that (29%) reported by a Canadian case-ascertained household transmission study of 13 asymptomatic pediatric index cases [5]. However, that study used systematic antibody testing, thereby enabling the identification of asymptomatic cases. Our estimate is also lower than reported in a Spanish study with only 36 household contacts (47%) [8] but exceeds that reported in a German study of 20 children (2%) [7].
While some reports have concluded that symptomatic pediatric index cases contribute more to household transmission than their asymptomatic counterparts [7, 10], others have reported that the risk does not differ between these groups [5, 32]. A living systematic review concluded that household SARs from asymptomatic index cases are approximately one-third of those from symptomatic index cases [33]. As in our study, a small study from China reported that pre-symptomatic index cases were associated with increased household transmission compared with asymptomatic index cases [34].
Our finding that younger SARS-CoV-2–infected children are associated with higher household SARs has been suggested previously [6, 32, 35, 36]. Interestingly, 2 studies that looked at household transmission across pediatric and adult age groups found that young children were one of the populations that contributed most to household transmission [6, 35]. This may be due to prolonged duration of viral shedding in the very young, as previously reported for children less than 3 years of age [9]. It may also be from closer contact due to their care needs, and a reduced ability to adhere to control measures (eg, mask wearing) among very young children. Finally, our finding of a higher SAR from asymptomatic children enrolled during periods of Delta and Omicron VoC circulation aligns with prior reports [36–38]. However, it is unclear if this finding indicates increased transmissibility of those SARS-CoV-2 variants or changes to isolation and preventive behaviors when compared with earlier pandemic time periods. In adjusted analysis, we did not find an association of household contact relationship to the index child (eg, parent vs sibling) and likelihood of becoming a secondary case, although others have found an association [5, 39]; this requires further evaluation as it has public health implications.
We observed an 8% risk of PCC 90 days after asymptomatic pediatric SARS-CoV-2 infection and the risk was greatest among adolescents, a finding previously reported in symptomatic children [19]. This estimate is comparable to another prospective cohort study, which found that 4% of 113 asymptomatic SARS-CoV-2–infected children reported PCC after 3 months [19]. Three other prospective studies reported that the PCC risk among asymptomatic children ranged from 0% to 27% [17, 18, 20]. All children reporting PCC in our study remained asymptomatic throughout the initial 14-day follow-up period; incident symptoms following the acute phase of SARS-CoV-2 illness was also observed in a prospective study of infected children in the United Kingdom [40]. Moreover, there are reports describing lung and other organ damage in individuals who were infected but asymptomatic during the acute SARS-CoV-2 infection phase [41].
Our study, to our knowledge, is the largest published pediatric asymptomatic SARS-CoV-2 household study with active prospective follow-up, which allowed us to analyze risk factors associated with household transmission. Additionally, the inclusion of similar, but unexposed “control” households allowed us to contextualize our SAR findings, which may have otherwise been considered nonspecific to SARS-CoV-2, by enabling us to incorporate the background clinical attack rate into our analyses. However, households with a SARS-CoV-2–positive index child would have been more vigilant to report their symptoms, which may have inflated their clinical SAR. Our estimate of the combined SARs has multiple limitations: we did not systematically test household contacts for SARS-CoV-2 infection during follow-up (ie, underestimating SARs) and positive tests reported to us may have been either via antigen or NAAT. Importantly, our analytic approach implies, but does not assign with certainty, the directionality of the SAR. Furthermore, some participants were hospitalized, reducing household contact exposure and possibly the SAR. A large proportion of pediatric participants had chronic underlying illnesses, which limits the generalizability of our findings. Last, the interpretation of our findings regarding PCC in SARS-CoV-2–positive children is limited by our lack of an asymptomatic, uninfected pediatric control group with 90-day follow-up. In a similar prospective cohort study recruiting SARS-CoV-2–tested symptomatic and asymptomatic children, PCC-type symptoms were also reported at a relatively high frequency for SARS-CoV-2–negative children; however, this occurred among a greater proportion of SARS-CoV-2–positive participants [19].
In conclusion, we found that asymptomatic SARS-CoV-2–infected children, especially those younger than 5 years and pre-symptomatic, are important sources of transmission within households, with 11% of household contacts developing symptoms within 2 weeks. We determined that the risk of developing symptomatic illness within 14 days was 5 times greater when contacts were exposed to an asymptomatic SARS-CoV-2–positive child in their household. The fact that approximately 1 in 13 asymptomatically infected children developed PCC is concerning and requires further investigation.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Supplementary Material
Contributor Information
Anna Funk, Department of Obstetrics and Gynecology, Cumming School of Medicine, University of Calgary, Calgary, Alberta, Canada.
Todd A Florin, Department of Pediatrics, Feinberg School of Medicine, Northwestern University, Chicago, Illinois, USA; Division of Emergency Medicine, Ann and Robert H. Lurie Children's Hospital Chicago, Chicago, Illinois, USA.
Nathan Kuppermann, Department of Emergency Medicine, University of California, Davis School of Medicine, Sacramento, California, USA; Department of Pediatrics, University of California, Davis School of Medicine, Sacramento, California, USA.
Yaron Finkelstein, Divisions of Emergency Medicine and Clinical Pharmacology and Toxicology, Department of Pediatrics, Hospital for Sick Children, University of Toronto, Toronto, Ontario, Canada.
Alissa Kazakoff, Faculty of Kinesiology, University of Calgary, Calgary, Alberta, Canada.
Michael Baldovsky, Division of Pediatric Emergency Medicine, University of Texas Southwestern Medical Center, Dallas, Texas, USA.
Daniel J Tancredi, Department of Pediatrics, University of California, Davis School of Medicine, Sacramento, California, USA.
Kristen Breslin, Division of Emergency Medicine, Children's National Hospital, Washington, D.C., USA.
Kelly R Bergmann, Department of Pediatric Emergency Medicine, Children's Minnesota, Minneapolis, Minnesota, USA.
Michael Gardiner, Department of Pediatrics, University of California, San Diego School of Medicine, San Diego, California, USA; Division of Emergency Medicine, Rady Children's Hospital, San Diego, California, USA.
Christopher M Pruitt, Department of Pediatrics, Medical University of South Carolina, Charleston, South Carolina, USA.
Deborah R Liu, Division of Emergency Medicine, Department of Pediatrics, Children's Hospital Los Angeles, Keck USC School of Medicine, Los Angeles, California, USA.
Mark I Neuman, Department of Pediatrics, Harvard Medical School, Boston, Massachusetts, USA; Division of Emergency Medicine, Boston Children's Hospital, Boston, Massachusetts, USA.
Matthew Wilkinson, Department of Pediatrics, University of Texas at Austin, Dell Medical School, Austin, Texas, USA.
Lilliam Ambroggio, Department of Pediatrics, University of Colorado, Aurora, Colorado, USA; Section of Emergency Medicine, Children's Hospital Colorado, Aurora, Colorado, USA.
Xiao-Li Pang, Department of Laboratory Medicine and Pathology, University of Alberta, Edmonton, Alberta, Canada.
Simon Cauchemez, Mathematical Modelling of Infectious Diseases Unit, Institut Pasteur, Université Paris Cité, CNRS UMR 2000, Paris, France.
Richard Malley, Division of Infectious Diseases, Boston Children's Hospital and Harvard Medical School, Boston, Massachusetts, USA.
Terry P Klassen, Children's Hospital Research Institute of Manitoba, University of Manitoba, Winnipeg, Manitoba, Canada; Department of Pediatrics and Child Health, University of Manitoba, Winnipeg, Manitoba, Canada.
Bonita E Lee, Department of Pediatrics, University of Alberta, Edmonton, Alberta, Canada.
Daniel C Payne, National Center for Emerging and Zoonotic Infectious Diseases, Centers for Disease Control and Prevention, Atlanta, Georgia, USA.
Salaheddin M Mahmud, Dept of Community Health Sciences, University of Manitoba, Winnipeg, Manitoba, Canada.
Stephen B Freedman, Section of Pediatric Emergency Medicine, Department of Pediatrics, Cumming School of Medicine, University of Calgary, Calgary, Alberta, Canada; Section of Gastroenterology, Department of Pediatrics, Cumming School of Medicine, University of Calgary, Calgary, Alberta, Canada; Department of Emergency Medicine, Cumming School of Medicine, University of Calgary, Calgary, Alberta, Canada.
Notes
Author Contributions. A. F., T. A. F., N. K., and S. B. F. obtained funding for the study. A. F., T. A. F., N. K., T. P. K., B. E. L., S. M. M., R. M., M. I. N., X.-L. P., D. C. P., D. J. T., S. C., and S. B. F. designed the study. X.-L. P. conducted laboratory analysis. A. K., Y. F., M. B., K. B., K. R. B., M. G., C. M. P., D. R. L., M. I. N., M. W., and L. A. collected the data. A. F. and D. J. T. performed the data analysis. A. F. and S. B. F. drafted the manuscript. All authors revised the manuscript and approved the final version.
Acknowledgments. The authors acknowledge the assistance of all our site research coordinators and assistants without whom this study would not have been possible and for the willingness of the families to participate in the study which enabled us to collect the necessary data to conduct our analyses. They also thank Yuanyuan Qiu for her technical support in performing viral load and variant of concern testing.
Financial support. This work was supported by the Canadian Institutes of Health Research (Operating Grant—COVID-19 Rapid Research Funding Opportunity; A. F., T. A. F., N. K., S. B. F.) and the COVID-19 Research Accelerator Funding Track (CRAFT) Program at the University of California, Davis (N. K.). S. B. F. is supported by the Alberta Children's Hospital Foundation Professorship in Child Health and Wellness. A. F. was supported by a University of Calgary Eye's High Postdoctoral Research fund as well as a Canadian Institutes of Health Research Banting Postdoctoral Fellowship.
Data availability. Deidentified data are available upon request from the principal investigator of the study (S. B. F.; Stephen.freedman@albertahealthservices.ca); however, evidence of ethics approval for use of the data will be required as well as contracts from both parties permitting data sharing. There must be a valid scientific purpose for use of the data and the request will need to be reviewed and approved by the study's leadership team.
References
- 1. Zhu Y, Bloxham CJ, Hulme KD, et al. A meta-analysis on the role of children in severe acute respiratory syndrome coronavirus 2 in household transmission clusters. Clin Infect Dis 2021; 72:e1146–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Waltenburg MA, Whaley MJ, Chancey RJ, et al. Household transmission and symptomology of severe acute respiratory syndrome coronavirus 2 Alpha variant among children—California and Colorado, 2021. J Pediatr 2022; 247:29–37.e27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Ng OT, Koh V, Chiew CJ, et al. Impact of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) vaccination and pediatric age on Delta variant household transmission. Clin Infect Dis 2022; 75:e35–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Donnelly MAP, Chuey MR, Soto R, et al. Household transmission of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) Alpha variant—United States, 2021. Clin Infect Dis 2022; 75:e122–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Bhatt M, Plint AC, Tang K, et al. Household transmission of SARS-CoV-2 from unvaccinated asymptomatic and symptomatic household members with confirmed SARS-CoV-2 infection: an antibody-surveillance study. CMAJ Open 2022; 10:E357–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Telle K, Jorgensen SB, Hart R, Greve-Isdahl M, Kacelnik O. Secondary attack rates of COVID-19 in Norwegian families: a nation-wide register-based study. Eur J Epidemiol 2021; 36:741–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Schumm L, Blankenburg J, Kahre E, et al. Lower SARS-CoV-2 household transmission in children and adolescents compared to adults. Sci Rep 2022; 12:22453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Soriano-Arandes A, Gatell A, Serrano P, et al. Household severe acute respiratory syndrome coronavirus 2 transmission and children: a network prospective study. Clin Infect Dis 2021; 73:e1261–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Liu Y, Xu L, Piao X, et al. Epidemiological, clinical, and household transmission characteristics of children and adolescents infected with SARS-CoV-2 omicron variant in Shanghai, China: a retrospective, multicenter observational study. Int J Infect Dis 2023; 129:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Chu VT, Yousaf AR, Chang K, et al. Household transmission of SARS-CoV-2 from children and adolescents. N Engl J Med 2021; 385:954–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Yu W, Guo Y, Zhang S, Kong Y, Shen Z, Zhang J. Proportion of asymptomatic infection and nonsevere disease caused by SARS-CoV-2 omicron variant: a systematic review and analysis. J Med Virol 2022; 94:5790–801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Sah P, Fitzpatrick MC, Zimmer CF, et al. Asymptomatic SARS-CoV-2 infection: a systematic review and meta-analysis. Proc Natl Acad Sci USA 2021; 118:e2109229118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Ma Q, Liu J, Liu Q, et al. Global percentage of asymptomatic SARS-CoV-2 infections among the tested population and individuals with confirmed COVID-19 diagnosis: a systematic review and meta-analysis. JAMA Netw Open 2021; 4:e2137257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Garrett N, Tapley A, Andriesen J, et al. High asymptomatic carriage with the omicron variant in South Africa. Clin Infect Dis 2022; 75:e289–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Murray CJL. COVID-19 will continue but the end of the pandemic is near. Lancet 2022; 399:417–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Ma Y, Deng J, Liu Q, Du M, Liu M, Liu J. Long-term consequences of asymptomatic SARS-CoV-2 infection: a systematic review and meta-analysis. Int J Environ Res Public Health 2023; 20:1613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Buonsenso D, Munblit D, De Rose C, et al. Preliminary evidence on long COVID in children. Acta Paediatr 2021; 110:2208–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Morello R, Mariani F, Mastrantoni L, et al. Risk factors for post-COVID-19 condition (long COVID) in children: a prospective cohort study. EClinicalMedicine 2023; 59:101961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Funk AL, Kuppermann N, Florin TA, et al. Post-COVID-19 conditions among children 90 days after SARS-CoV-2 infection. JAMA Netw Open 2022; 5:e2223253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Say D, Crawford N, McNab S, Wurzel D, Steer A, Tosif S. Post-acute COVID-19 outcomes in children with mild and asymptomatic disease. Lancet Child Adolesc Health 2021; 5:e22–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Boyton RJ, Altmann DM. The immunology of asymptomatic SARS-CoV-2 infection: what are the key questions? Nat Rev Immunol 2021; 21:762–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Koelle K, Martin MA, Antia R, Lopman B, Dean NE. The changing epidemiology of SARS-CoV-2. Science 2022; 375:1116–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. American Academy of Pediatrics . COVID-19 hospital admissions among children rising again. AAP News. 19 September 2023. Available at: https://publications.aap.org/aapnews/news/26147/COVID-19-hospital-admissions-among-children-rising. Accessed 4 October 2023.
- 24. Agathis NT, Patel K, Milucky J, et al. Codetections of other respiratory viruses among children hospitalized with COVID-19. Pediatrics 2023; 151:e2022059037. [DOI] [PubMed] [Google Scholar]
- 25. Hasing ME, Lee BE, Gao T, et al. Wastewater surveillance monitoring of SARS-CoV-2 variants of concern and dynamics of transmission and community burden of COVID-19. Emerg Microbes Infect 2023; 12:2233638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Government of Canada . COVID-19 epidemiology update: summary. Updated 4 October 2023. Available at: https://health-infobase.canada.ca/covid-19/testing-variants.html. Accessed 4 October 2023.
- 27. Centers for Disease Control and Prevention . COVID data tracker. Atlanta, GA: US Department of Health and Human Services, Centers for Disease Control and Prevention, 2023. [Google Scholar]
- 28. World Health Organization . A clinical case definition for post COVID-19 condition in children and adolescents by expert consensus, 16 February 2023. Available at: https://www.who.int/publications/i/item/WHO-2019-nCoV-Post-COVID-19-condition-CA-Clinical-case-definition-2023-1. Accessed 4 October 2023.
- 29. Cauchemez S, Donnelly CA, Reed C, et al. Household transmission of 2009 pandemic influenza A (H1N1) virus in the United States. N Engl J Med 2009; 361:2619–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Cauchemez S, Carrat F, Viboud C, Valleron AJ, Boëlle PY. A Bayesian MCMC approach to study transmission of influenza: application to household longitudinal data. Stat Med 2004; 23:3469–87. [DOI] [PubMed] [Google Scholar]
- 31. Zou G. A modified Poisson regression approach to prospective studies with binary data. Am J Epidemiol 2004; 159:702–6. [DOI] [PubMed] [Google Scholar]
- 32. Paul LA, Daneman N, Schwartz KL, et al. Association of age and pediatric household transmission of SARS-CoV-2 infection. JAMA Pediatr 2021; 175:1151–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Buitrago-Garcia D, Ipekci AM, Heron L, et al. Occurrence and transmission potential of asymptomatic and presymptomatic SARS-CoV-2 infections: update of a living systematic review and meta-analysis. PLoS Med 2022; 19:e1003987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Zhang W, Cheng W, Luo L, et al. Secondary transmission of coronavirus disease from presymptomatic persons, China. Emerg Infect Dis 2020; 26:1924–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Bistaraki A, Roussos S, Tsiodras S, Sypsa V. Age-dependent effects on infectivity and susceptibility to SARS-CoV-2 infection: results from nationwide contact tracing data in Greece. Infect Dis (Lond) 2022; 54:186–95. [DOI] [PubMed] [Google Scholar]
- 36. Galmiche S, Charmet T, Rakover A, et al. Risk of SARS-CoV-2 infection among households with children in France, 2020–2022. JAMA Netw Open 2023; 6:e2334084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Jalali N, Brustad HK, Frigessi A, et al. Increased household transmission and immune escape of the SARS-CoV-2 Omicron compared to Delta variants. Nat Commun 2022; 13:5706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Madewell ZJ, Yang Y, Longini IM Jr, Halloran ME, Dean NE. Household secondary attack rates of SARS-CoV-2 by variant and vaccination status: an updated systematic review and meta-analysis. JAMA Netw Open 2022; 5:e229317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Laxminarayan R, Wahl B, Dudala SR, et al. Epidemiology and transmission dynamics of COVID-19 in two Indian states. Science 2020; 370:691–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Stephenson T, Pinto Pereira SM, Nugawela MD, et al. Long COVID—six months of prospective follow-up of changes in symptom profiles of non-hospitalised children and young people after SARS-CoV-2 testing: a national matched cohort study (the CLoCk) study. PLoS One 2023; 18:e0277704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Long QX, Tang XJ, Shi QL, et al. Clinical and immunological assessment of asymptomatic SARS-CoV-2 infections. Nat Med 2020; 26:1200–4. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.