Abstract
Objective:
To investigate the oncological outcomes after transanal total mesorectal excision (TaTME) for rectal cancer and risk factors for local recurrence (LR).
Background:
A high LR rate with a multifocal pattern early after TaTME has been reported in Norway and the Netherlands, causing controversy over the oncological safety of this technique.
Methods:
Twenty-six member institutions of the Japan Society of Laparoscopic Colorectal Surgery participated in this retrospective cohort study. A total of 706 patients with primary rectal cancer who underwent TaTME between January 2012 and December 2019 were included for analysis. The primary endpoint was the cumulative 3-year LR rate.
Results:
A total of 253 patients had clinical stage III disease (35.8%) and 91 (12.9%) had stage IV. Intersphincteric resection was performed in 318 patients (45.0%) and abdominoperineal resection in 193 (27.3%). There was 1 urethral injury (0.1%). A positive resection margin (R1) was seen in 42 patients (5.9%). Median follow-up was 3.42 years, and the 2- and 3-year cumulative LR rates were 4.95% (95% confidence interval: 3.50–6.75) and 6.82% (95% confidence interval: 5.08–8.89), respectively. A multifocal pattern was observed in 14 (25%) of 56 patients with LR. Tumor height from the anal verge, pathological T4 disease, pathological stage III/IV, positive perineural invasion, and R1 resection were significant risk factors for LR in multivariable analysis.
Conclusions:
In this selected cohort in which intersphincteric resection or abdominoperineal resection was performed in more than half of cases, oncological outcomes were acceptable during a median follow-up of more than 3 years.
Keywords: local recurrence, rectal cancer, TaTME, transanal total mesorectal excision
Mini abstract:
This retrospective cohort study included 706 patients with rectal cancer who underwent transanal total mesorectal excision. Intersphincteric or abdominoperineal resection was performed in more than half of the cases. The median follow-up period was 3.42 years, and the 3-year cumulative local recurrence rate was 6.82%.
INTRODUCTION
After first being reported in 2010,1 the novelty and innovativeness of transanal total mesorectal excision (TaTME) resulted in its rapid adoption worldwide, especially for mid and low rectal cancer. However, serious concerns regarding the oncological safety of this procedure were raised by studies conducted in Norway and the Netherlands in 2020.2,3 Their findings of a high local recurrence (LR) rate of 10.0%–11.6% with a multifocal pattern early after TaTME have caused controversy over this technique worldwide and the introduction of a moratorium on performing it in Norway. A limited number of multicenter large-scale cohort studies have investigated the concerns about TaTME4–7 and demonstrated oncological outcomes that are comparable with those of the recent large randomized studies of conventional TME.8,9 However, more data are required because currently available data do not consistently support the safety of TaTME. Importantly, the available data were obtained from high-volume centers in Western countries or from the International TaTME Registry. Furthermore, most of the patients in those studies underwent low anterior resection (AR), with very few undergoing abdominoperineal resection (APR) or intersphincteric resection (ISR).
Compared with transabdominal TME,10 the potential benefit of TaTME is the ability to perform meticulous and stable dissection in the deep pelvis owing to superior visualization and maneuverability, which would possibly be maximized by ISR or APR. However, the indications and criteria for TaTME vary from country to country. ISR has been performed widely for very low rectal cancer11,12 and is considered a good indication for TaTME in a number of institutions in Japan.13,14 Furthermore, a considerable number of colorectal surgeons in Japan consider TaTME to be a good option for APR. By contrast, the negative aspects of TaTME, including complications such as urethral injury and potentially unfavorable oncological outcomes, might become more evident in such situations. However, nationwide Japanese data on the clinical and oncological outcomes of TaTME are not available as yet.
Therefore, we performed this multicenter cohort study to investigate the oncological safety of a minimally invasive transanal approach for rectal cancer in Japan (the Ta-Ta-Mi study). Risk factors for LR were also assessed.
METHODS
Study Population
Patients with primary rectal cancer who underwent TaTME at any of 26 Japan Society of Laparoscopic Colorectal Surgery member institutions between January 2012 and December 2019 were retrospectively identified. The inclusion criteria were a histological diagnosis of adenocarcinoma or adenosquamous carcinoma, a lower tumor margin within 12 cm from the anal verge (AV), and clinical stage I–IV disease. Patients with recurrent disease, multiple cancers, or ulcerative colitis and those who underwent concomitant surgery for another disease were excluded. Tumors were classified according to the American Joint Committee on Cancer tumor-node-metastasis system.15 All data were collected electronically via the Research Electronic Data Capture system.
The study was approved by the Institutional Review Board and Ethics Committee of Kobe University Graduate School of Medicine (IRB reference code: B220092). The need for informed consent was waived in view of the anonymity of the study data. However, in accordance with the Japanese ethical regulations, Japanese Personal Information Protection Law, and instructions of the Ethics Committees of each institution, consent was secured via the opt-out route. Information regarding the purpose and methodology of the study was disclosed on the hospital noticeboard and/or website and opportunities to opt out were provided to the extent possible.
Endpoints
The primary endpoint was the cumulative 3-year LR rate. Secondary endpoints included the pattern of LR, positive radial and distal margin (DM) rates, incidence of intraoperative adverse events, incidence of postoperative complications that were grade ≥3 according to the Clavien–Dindo classification,16 the 3-year relapse-free survival rate, and the 3-year overall survival rate.
Definitions
LR was defined as any recurrent disease in the pelvis or at the anastomotic site that was confirmed by radiological or endoscopic examination. Distant recurrence was defined as any recurrence outside of the pelvic cavity. The radial margin (RM) according to the Japanese Classification of Colorectal, Appendiceal, and Anal Carcinoma17 was evaluated in some institutions instead of circumferential resection margin (CRM). A positive (C)RM or DM was defined as the presence of a tumor or malignant lymph nodes at ≤1 mm from the resection margin. The pathological tumor response to neoadjuvant therapy was determined based on the grading scale according to the Japanese Classification of Colorectal, Appendiceal, and Anal Carcinoma.17 Briefly, grades 0, 1a, 1b, 2, and 3 correspond to no response to treatment, 1/3 tumor size reduction, 1/3–2/3 tumor size reduction, >2/3 tumor size reduction, and complete tumor ablation, respectively. The interrupted suture to close the rectum for ISR was classified into double or more purse-string sutures. Purse-string suture failure was defined as any leakage from the closed rectal lumen during dissection.
Procedures
Although TaTME was basically performed following the standardized procedure described by Lacy et al,10 the criteria for TaTME, choice of a 1- or 2-team approach, and choice of transabdominal approach were at the discretion of the individual institution.
After insertion of a single-port device such as a GelPOINT path, the rectal lumen was closed with ≥1 purse-string sutures. After lavage of the rectum, rectotomy was started. For ISR, whether a purse-string or interrupted suture method was used to close the rectum and whether rectotomy was started before or after closure of the rectum was decided by the individual surgeon. For APR, the circumferential skin incision around the anus was started after closure of the anus by single or double purse-string sutures.
Follow-Up
Patients were followed up in accordance with the guidelines of the Japanese Society for Cancer of the Colon and Rectum.18 Briefly, follow-up was performed every 3 months for the first 3 years and at 6-month intervals thereafter. Each follow-up included a physical examination and measurement of tumor markers, including carcinoembryonic antigen and carbohydrate antigen 19–9. Computed tomography was performed every 6 months at least for the first 3 years and annually thereafter. Total colonoscopy every 2 years was recommended.
Statistical Analysis
Continuous variables are shown as the median and interquartile range (IQR) and categorical variables as the number and proportion, including cases with missing values in the denominator. The cumulative incidence of LR was calculated while accounting for competing risks (ie, systemic recurrence and death). Relapse-free survival and overall survival rates were calculated using the Kaplan–Meier method. Cause-specific Cox proportional hazards regression was used to analyze the association between time to LR and potential risk factors for LR. Continuous variables (ie, distance from the AV, carcinoembryonic antigen level, number of TaTMEs performed by the surgeon, and hospital TaTME volume) were checked for a nonlinear association with the outcome using penalized smoothing splines. If a nonlinear term was found to be nonsignificant, the variable was included as a linear term in the regression. Potential risk factors were identified by univariable regression analysis and entered into the final multivariable regression model in a stepwise manner based on the Akaike information criterion. Missing values were imputed with multiple imputation using the mice package in R.19 Twenty datasets were generated with missing values imputed. The above-mentioned variable selection procedure was repeated for the imputed datasets, and the majority rule and Wald test were applied to choose the variables for the final model.20 The correlation between scaled Schoenfeld residuals and time was tested if the proportional hazards assumption was met for each variable included in the model. All statistical analyses were performed using R version 4.2.2 (R Foundation for Statistical Computing, Vienna, Austria, 2022). A 2-sided P < 0.05 was considered statistically significant.
RESULTS
A total of 706 patients with rectal cancer who underwent TaTME at any of the 26 participating institutions were eligible for inclusion in the analysis. The patient and tumor characteristics are shown in Table 1. Of these patients, 488 (69.1%) were male and 392 (55.5%) did not receive neoadjuvant therapy. The median tumor height from the AV was 4.0 cm (IQR: 3.0, 5.0). Approximately half of the tumors (56.4%) were located at the anterior wall or were circumferential. In addition, 253 patients (35.8%) had clinical stage III disease and 91 (12.9%) had clinical stage IV disease, and 267 patients (37.8%) received adjuvant therapy.
TABLE 1.
Patient and Tumor Characteristics
| Variable | Total Cohort |
|---|---|
| (N = 706) | |
| Age, median (IQR) | 66 (57, 72) |
| Sex, n (%) | |
| Male | 488 (69.1) |
| Female | 218 (30.9) |
| BMI, n (%) | |
| Normal (18.5–24.9) | 430 (60.9) |
| Underweight (<18.5) | 78 (11.0) |
| Overweight (25.0–29.9) | 170 (24.1) |
| Obese (≥30.0) | 28 (4.0) |
| ASA score, n (%) | |
| I | 209 (29.6) |
| II | 444 (62.9) |
| III | 52 (7.4) |
| IV | 1 (0.1) |
| Neoadjuvant therapy, n (%) | |
| None | 392 (55.5) |
| NACRT | 106 (15.0) |
| NAC | 188 (26.6) |
| RT | 8 (1.1) |
| TNT | 12 (1.7) |
| Distance from AV (cm), median (IQR) | 4.0 (3.0, 5.0) |
| Tumor location, n (%) | |
| Anterior/circumferential | 398 (56.4) |
| Not anterior | 308 (43.6) |
| cT*, n (%) | |
| 0/is | 1 (0.3) |
| 1 | 106 (15.0) |
| 2 | 128 (18.1) |
| 3 | 334 (47.3) |
| 4a | 59 (8.4) |
| 4b | 75 (10.6) |
| cN*, n (%) | |
| 0 | 381 (54.0) |
| 1 | 198 (28.0) |
| 2 | 127 (18.0) |
| cM*, n (%) | |
| 0 | 610 (86.4) |
| 1 | 96 (13.6) |
| cStage*, n (%) | |
| I | 205 (29.0) |
| II | 157 (22.2) |
| III | 253 (35.8) |
| IV | 91 (12.9) |
| CEA, median (IQR) | 3.7 (2.2, 7.4) |
| Missing, n (%) | 1 (0.1) |
| CA19-9, median (IQR) | 10.7 (6.0, 21.0) |
| Missing, n (%) | 3 (0.4) |
| Adjuvant chemotherapy, n (%) | |
| No | 439 (62.2) |
| Yes | 268 (37.8) |
Tumors were classified according to the American Joint Committee on Cancer TNM system.
ASA indicates American Society of Anesthesiologists; AV, anal verge; BMI, body mass index; CA19-9, carbohydrate antigen 19-9; CEA, carcinoembryonic antigen; IQR, interquartile range; NAC, neoadjuvant chemotherapy; NACRT, neoadjuvant chemoradiotherapy; RT, radiotherapy; TNM, tumor-node-metastasis; TNT, total neoadjuvant therapy.
Operative and postoperative outcomes are shown in Table 2. ISR was performed in 318 patients (45.0%) and APR in 193 (27.3%). Lateral pelvic lymph node dissection was performed unilaterally in 78 patients (11.0%) and bilaterally in 159 (22.5%). Robotic surgery was used for the abdominal approach in 126 patients (17.8%). In the 472 patients (66.9%) in whom an anastomosis was created, single stapling was performed in 147 (20.8%) and hand-sewn sutures were used in 325 (46.0%). At least double purse-string sutures were used in 545 patients (77.2%) to close the rectum. In terms of intraoperative adverse events during transanal dissection, purse-string failure occurred in 14 patients (2.0%), rectal perforation in 11 (1.6%), and vaginal perforation in 6 (0.8%). Urethral injury occurred in 1 patient (0.1%). Clavien–Dindo grade ≥2 and 3 postoperative complications occurred in 173 patients (24.5%) and 90 patients (12.7%), respectively.
TABLE 2.
Operative and Postoperative Outcomes
| Variable | Total Cohort |
|---|---|
| N = 706 | |
| Operative procedure, n (%) | |
| Anterior resection | 166 (23.5) |
| Intersphincteric resection | 318 (45.0) |
| Abdominoperineal resection | 193 (27.3) |
| Hartmann | 25 (3.5) |
| Other | 4 (0.6) |
| Combined resection, n (%) | |
| Prostate | 8 (1.1) |
| Vagina | 13 (1.8) |
| Uterus | 4 (0.6) |
| Other | 54 (7.6) |
| Lymph node dissection*, n (%) | |
| prxD2 | 63 (8.9) |
| prxD3 | 643 (91.1) |
| LLND, n (%) | |
| Unilateral | 78 (11.0) |
| Bilateral | 159 (22.5) |
| No | 469 (66.4) |
| Autonomic nerve resection, n (%) | |
| Unilateral | 35 (5.0) |
| Bilateral | 17 (2.4) |
| No | 654 (92.6) |
| Abdominal approach, n (%) | |
| Open | 10 (1.4) |
| Laparoscopic | 570 (80.7) |
| Robotic | 126 (17.8) |
| Diverting stoma, n (%) | 425 (60.2) |
| Type of anastomosis, n (%) | |
| None | 219 (31.0) |
| SST | 147 (20.8) |
| Hand-sewn | 325 (46.0) |
| Other | 15 (2.1) |
| Operation time (min), median (IQR) | 412 (310, 537) |
| Estimated blood loss (g), median (IQR) | 50 (10, 120) |
| Transfusion, n (%) | |
| Yes | 25 (3.5) |
| No | 681 (96.5) |
| Purse-string suture†, n (%) | |
| Single | 157 (22.2) |
| Double or more | 545 (77.2) |
| Missing | 4 (0.6) |
| Adverse events during TaTME, n (%) | |
| Purse-string failure | 14 (2.0) |
| Visceral injury | |
| Rectum | 11 (1.6) |
| Vagina | 6 (0.8) |
| Urethra | 1 (0.1) |
| Autonomic nerve | 3 (0.4) |
| Bleeding | 4 (0.6) |
| Conversion | 3 (0.4) |
| Other | 5 (0.7) |
| Postoperative complications, n (%) | |
| CD grade ≥2 | 173 (24.5) |
| CD grade ≥3 | 90 (12.7) |
According to the Japanese Classification of Colorectal, Appendiceal, and Anal Carcinoma.
The interrupted suture to close the rectum for intersphincteric resection was classified into double or more purse-string sutures.
CD indicates Clavien–Dindo classification; IQR, interquartile range; LLND, lateral pelvic lymph node dissection; SST, single stapling technique; TaTME, transanal total mesorectal excision.
Table 3 shows the pathological outcomes. The median tumor diameter was 32 mm (IQR: 20, 47). The number of patients with pathological stage III and IV disease was 182 (25.8%) and 72 (10.2%), respectively. Positive lateral pelvic lymph node metastasis was found in 55 patients (7.8%). The (C)RM was positive in 42 patients (5.9%) and unknown in 34 (4.8%). Positive resection status ([C]RM+ and/or DM+) was seen in 42 patients (5.9%). A good pathological response (grade 2 or 3) was obtained in 107 (34.1%) of the 314 patients who received neoadjuvant therapy.
TABLE 3.
Pathological Outcomes
| Variable | Total Cohort |
|---|---|
| N = 706 | |
| Histological type, n (%) | |
| Differentiated | 653 (92.5) |
| Undifferentiated | 39 (5.5) |
| Other | 14 (2.0) |
| Tumor diameter (mm), median (IQR) | 32 (20, 47) |
| Missing, n (%) | 2 (0.3) |
| (y)pT*, n (%) | |
| 0/is | 41 (5.8) |
| 1 | 131 (18.6) |
| 2 | 179 (25.4) |
| 3 | 304 (43.1) |
| 4a | 26 (3.7) |
| 4b | 25 (3.5) |
| (y)pN*, n (%) | |
| 0 | 472 (66.9) |
| 1 | 165 (23.4) |
| 2 | 69 (9.8) |
| (y)pM*, n (%) | |
| 0 | 633 (89.7) |
| 1 | 73 (10.3) |
| (y)pStage*, n (%) | |
| 0 | 45 (6.4) |
| I | 256 (36.3) |
| II | 151 (21.4) |
| III | 182 (25.8) |
| IV | 72 (10.2) |
| Positive LLN metastasis, n (%) | 55 (7.8) |
| Lymphatic invasion, n (%) | |
| Absent | 443 (62.7) |
| Present | 263 (37.3) |
| Vascular invasion, n (%) | |
| Absent | 318 (45.0) |
| Present | 388 (55.0) |
| Budding†, n (%) | |
| BD1 | 324 (45.9) |
| BD2 | 41 (5.8) |
| BD3 | 26 (3.7) |
| BDX | 315 (44.6) |
| Perineural invasion, n (%) | |
| Absent | 459 (65.0) |
| Present | 164 (23.2) |
| unknown | 83 (11.8) |
| (C)RM, n (%) | |
| Negative (>1 mm) | 630 (89.2) |
| Positive (≤1 mm) | 42 (5.9) |
| Unknown | 34 (4.8) |
| DM, n (%) | |
| Negative (>1 mm) | 700 (99.2) |
| Positive (≤1 mm) | 4 (0.6) |
| Unknown | 2 (0.3) |
| Resection status, n (%) | |
| R0 | 633 (89.2) |
| R1 | 42 (5.9) |
| RX | 35 (4.9) |
| Number of LNs harvested, median (range) | 17 (11, 27) |
| Pathological response†, n (%) | |
| Good | 107 (34.1) |
| Poor | 205 (65.3) |
| Missing | 2 (0.6) |
Tumors were classified according to the American Joint Committee on Cancer TNM system.
According to the Japanese Classification of Colorectal, Appendiceal, and Anal Carcinoma.
IQR indicates interquartile range; LLN, lateral pelvic lymph node; LNs, lymph nodes; LR, local recurrence; R1, positive (C)RM and/or positive DM; TNM, tumor-node-metastasis.
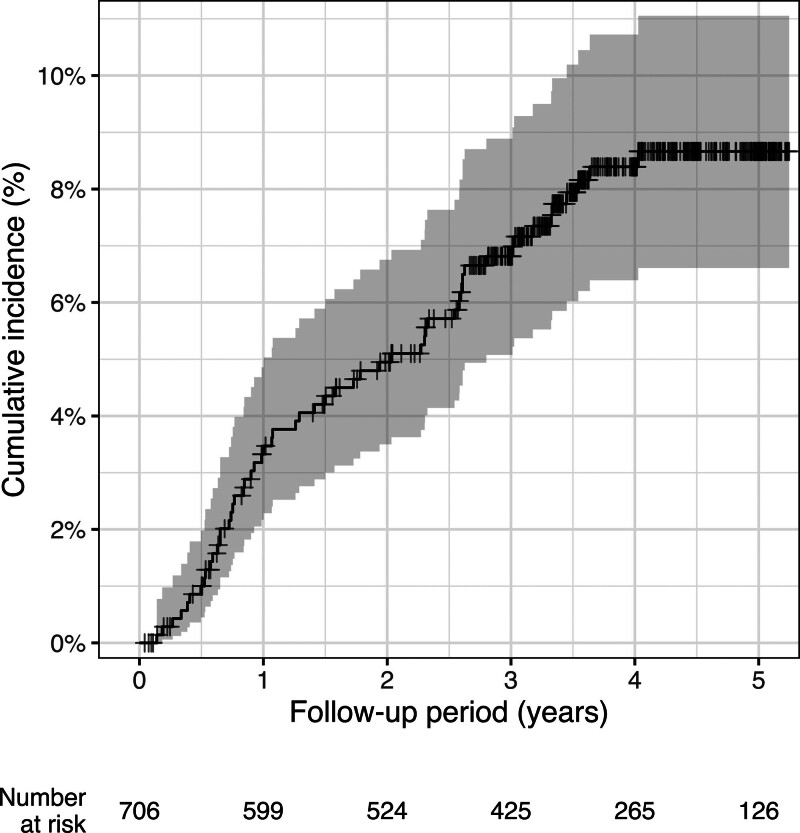
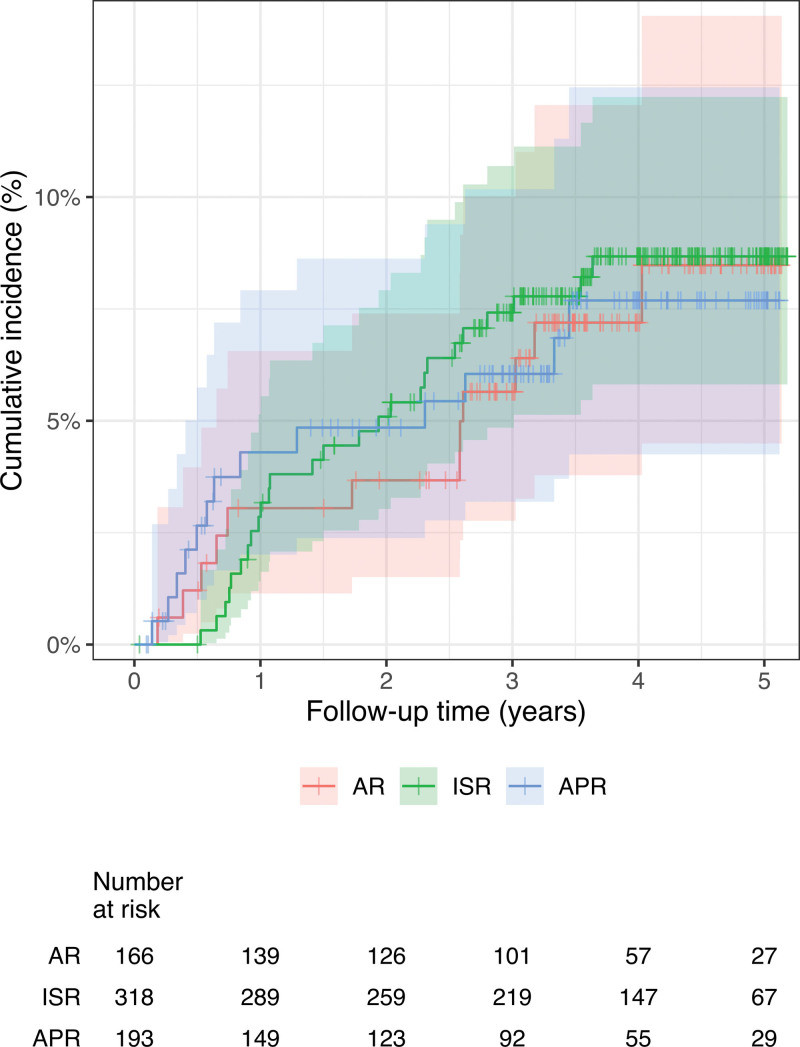
The oncological outcomes are shown in Table 4 and the cumulative incidence of LR is shown in Figure 1. The 2- and 3-year cumulative LR rates were 4.95% (95% confidence interval [CI]: 3.50–6.75) and 6.82% (95% CI: 5.08–8.89), respectively, during a median follow-up of 3.42 years. A multifocal pattern was observed in 14 (25%) of 56 patients with LR. The median time to LR was 1.35 years (IQR: 0.71, 2.59). The cumulative incidence of LR in AR, ISR, and APR is shown in Figure 2. There was no significant difference in the LR incidence between the groups.
Table 4.
Oncological Outcomes
| Variable | Total Cohort |
|---|---|
| N = 706 | |
| Median follow-up duration, years (IQR) | 3.42 (1.95, 4.62) |
| 2-year LR, % (95% CI) | 4.95 (3.50–6.75) |
| Central pelvis | 1.75 (0.96–2.95) |
| Lateral pelvis | 1.46 (0.75–2.58) |
| Anastomosis | 0.30 (0.06–1.01) |
| Multifocal | 1.45 (0.75–2.58) |
| 3-year LR, % (95% CI) | 6.82 (5.08–8.89) |
| Central pelvis | 2.53 (1.53–3.93) |
| Lateral pelvis | 2.07 (1.19–3.37) |
| Anastomosis | 0.45 (0.13–1.25) |
| Multifocal | 1.76 (0.96–2.97) |
| Pattern of LR, n (%) | |
| Unifocal | 42/56 (75.0) |
| Multifocal | 14/56 (25.0) |
| Time to LR (years), median (IQR) | 1.35 (0.71, 2.59) |
| 3-year RFS, % (95% CI) | 73.3 (70.0–76.7) |
| 3-year OS, % (95% CI) | 91.6 (89.5–93.7) |
CI indicates confidence interval; IQR, interquartile range; LR, local recurrence; OS, overall survival; RFS, relapse-free survival.
FIGURE 1.
Cumulative incidence of local recurrence after surgery. The respective 2-year and 3-year cumulative local recurrence rates were 4.95% and 6.82%.
FIGURE 2.
Cumulative incidence of local recurrence in AR, ISR, and APR. The 3-year cumulative local recurrence rates in AR, ISR, and APR were 5.65%, 7.42%, and 6.05%, respectively.
The results of univariable and multivariable analyses of potential risk factors for LR are shown in Supplemental Table 1 http://links.lww.com/AOSO/A286. Continuous variables did not have a significant nonlinear association with the outcome in the regression analysis and so were included as a linear term. The final multivariable regression model included tumor height from AV, pathological T stage, pathological stage, operative procedure, perineural invasion, and resection status. Neither the number of TaTMEs performed by the surgeon nor the hospital TaTME volume were selected based on the Akaike information criterion. Finally, tumor height from the AV, pathological T4 disease, pathological stage III/IV, perineural invasion, and R1 resection were identified to be significant predictors of LR.
DISCUSSION
The oncological safety of TaTME has been a major ongoing concern because of the reports of high rates of LR with a multifocal pattern from Norway and the Netherlands in 2020.2,3 A few large multicenter cohort studies have demonstrated an acceptable LR rate (3.0%–6.6%),4–7 but were performed at tertiary referral centers in Western countries or were based on International TaTME Registry data with median follow-up periods of only 24–27 months. In this study, the 2- and 3-year cumulative LR rates were 4.95% (95% CI: 3.50–6.75) and 6.82% (95% CI: 5.08–8.89), respectively, during a median follow-up of 3.42 years (95% CI: 1.95–4.62). The median time to LR was 1.35 years (95% CI: 0.71–2.59), and a quarter of the LRs had a multifocal pattern. The median number of TaTME procedures performed per year by the 26 participating member institutions of the Japan Society of Laparoscopic Colorectal Surgery was 15 (IQR: 9, 22) during the study period. Our study is the first nationwide Japanese investigation of the surgical and oncological outcomes of TaTME, and had a median follow-up of ≥3 years.
Although our 2-year LR rate was similar to that of the International TaTME Registry,5 it was arguably somewhat worse than that in other multicenter cohort studies.4,6 However, one of the important features of the present study is that the proportion of patients who underwent ISR (45.0%) was much higher than that in other large-scale cohort studies despite the similarity in tumor height from the anus. Our data indicate that the TaTME approach is more suitable for ISR than for LAR in Japan. ISR is the ultimate anal-sparing alternative to APR, but it is known to be associated with a high LR rate.21,22 In a retrospective single-center study from South Korea, LR occurred in 18 (11.2%) of 161 patients who underwent conventional ISR during a median follow-up of 55 months.22 In that study, the 3-year LR-free survival rate was 89%. A multicenter Phase II clinical trial that included 8 top-tier Japanese institutions and 110 patients with T1–T3 rectal cancer reported a 3-year LR rate of 13.2%.23 Therefore, our 2- and 3-year LR rates seem to be comparable with those of previous studies, suggesting that TaTME could be a useful option for ISR. Importantly, the rate of neoadjuvant therapy was only 44.5% in this study, because neoadjuvant therapy has not been recommended for patients with resectable rectal cancer according to the guidelines of the Japanese Society for Cancer of the Colon and Rectum.18 Greater use of neoadjuvant therapy could further decrease the LR rate in our cohort.
This study also included a higher proportion of patients who underwent APR (27.3%). Although the risk of recurrence is considered to be higher after APR than after AR,24–26 we found the hazard ratio for LR to be significantly lower than that for AR. One possible reason for this finding may be that the TaTME approach enables good control of perineal and levator ani muscle dissection. Holm et al27 developed the extralevator abdominoperineal excision procedure in which the levator ani muscle is divided as laterally as possible to allow more cylindrical resection and reported that the oncological outcomes were better with this technique than with standard APR. Mège et al28 reported that the primary perineal approach for APR had promising surgical and oncological outcomes with advantages similar to those of TaTME, including retrograde dissection with control of levator ani insertions, a smaller incision, and no need for a change in position. Further analyses are necessary to clarify the possible advantages of the TaTME approach when performing APR.
Another feature of our study is that the percentage of patients with stage IV disease was higher than in other studies.4–6 In the International TaTME Registry study, the 2-year LR rate was 11.1% (95% CI: 6.1–16.0) in patients with stage IV disease and 4.8% (95% CI: 3.8–5.8) in the entire cohort.5 Another study that included 767 patients, some of whom had data in the same International Registry, found that LR-free survival in patients with stage IV disease was no worse than that in other patients.6 However, it remains unclear whether patients with stage IV disease are at increased risk of LR because the major studies of laparoscopic vs. open TME for rectal cancer have tended not to include patients with stage IV disease.8,29,30 Furthermore, LR after TaTME in patients with stage IV disease may occur via a specific mechanism, such as spillage of tumor cells from blood vessels. Therefore, further data on stage IV cases treated by TaTME are needed.
It is well known that TaTME is associated with procedure-specific complications, particularly urethral injury. Sylla et al31 reported that 20 of 39 urological injuries with TaTME occurred early on the learning curve and that the risk of such injuries could be reduced by structured training and supervision. In our study, urethral injury occurred in 1 patient; this translates to a rate of 0.1%, which seems comparable with the rates in the previous large-scale cohort studies.4,7 Moreover, the rates of purse-string failure (2.0%) and rectal perforation (1.6%) in our study were similar to the rates of 2.5% and 1.6%, respectively, reported by the International TaTME Registry.5 These data suggest that TaTME has been successfully implemented nationwide in Japan. However, there is presently no nationwide skill qualification certification or quality assurance system for TaTME in Japan, and its implementation and indications are left to the individual surgeon or institution. Our data do not suggest that regulations, guidelines, and quality assurance are unnecessary for implementation of TaTME.
The Dutch and Norwegian learning curve data suggested that technical failure during implementation, such as purse-string failure, might cause early LR with a multifocal pattern.7,32 The study performed in the Netherlands investigated the LR rate in the first 10 TaTME cases performed at each of the 12 participating centers and found it to be 10.0% (ie, 12 LRs in 120 cases), with a multifocal pattern in 8 of the 12 LRs. According to their data, intraoperative complications represented the most significant risk factor for multifocal LR. In our study, occurrence of intraoperative adverse events, including purse-string failure and visceral injury, was not a significant risk factor for LR after TaTME. Similarly, we did not find the number of procedures performed by the individual surgeon to be a risk factor for LR; the median number in our study was 20 (IQR: 6, 41), which appears insufficient in view of previous research suggesting that the learning curve requires at least 40 cases.33–35 However, according to our data, limited experience is not in itself associated with a poor oncological outcome as long as the procedure is performed correctly and in appropriately selected patients. Further evidence in support of this view is our finding in univariate analysis that hospital volume was not a significant risk factor for LR (hazard ratio 1.00, 95% CI: 0.97–1.02).
This study has several limitations. First, because it was a multicenter retrospective cohort study, selection, reporting, and technical bias cannot be excluded. Furthermore, a higher proportion of patients who underwent APR were included. Because the technique of APR using TaTME approach has not been well discussed so far, it might be unfair to include those patients. Second, some institutions use the RM instead of the CRM, which is considered to be the most appropriate surrogate for successful resection. Considering that estimation of the CRM has not been popularized or standardized in Japan and use of RM is recommended by the Japanese Classification of Colorectal, Appendiceal, and Anal Carcinoma,17 we included R1 resection status as a potential risk factor for LR in the regression analysis. Third, the proportion of the patients receiving neoadjuvant therapy was small and the indication for lateral lymph node dissection varied by the institution. It would affect the oncological outcomes in this study.
In conclusion, this study was performed in a selected cohort of patients, half of whom underwent ISR or APR. Oncological outcomes were acceptable during a median follow-up of ≥3 years. The risk factors for LR seemed not to be learning curve-related, as suggested by data from Norway and the Netherlands. The results of the ongoing COLOR III and ETAP-GRECCAR 11 studies36,37 should allow more definitive conclusions regarding the oncological safety and validity of TaTME.
ACKNOWLEDGMENTS
We thank the Ta-Ta-Mi study group collaboratives in Japan Society of Laparoscopic Colorectal Surgery: Akihiro Kondo, Akinobu Taketomi, Akiyoshi Kanazawa, Atsushi Ogura, Chikayoshi Tani, Gunpei Yoshimatsu, Hideya Kashihara, Hirokazu Suwa, Kay Uehara, Keiichi Okano, Keiichiro Okuyama, Kenji Okita, Kiyoshi Maeda, Kohei Shigeta, Koji Okabayashi, Masafumi Inomata, Masaji Tani, Masataka Ikeda, Masatsune Shibutani, Masayoshi Iwamoto, Masayuki Ishii, Shigenori Honma, Nobuki Ichikawa, Shiro Terai, Takeshi Okabayashi, Tatsunari Fukuoka, Tatsuya Manabe, Tatsuya Shonaka, Toshikatsu Nitta, Tomonori Akagi, Toru Miyake, Toru Kuramoto, Yoshihito Ide, Yosuke Fukunaga, Yuki Murata, Yukinari Tokoro, Yukitoshi Todate, Yusaku Shogen.
Supplementary Material
Footnotes
Published online 8 January 2024
This work was supported by a grant from the Japanese Foundation for Research and Promotion of Endoscopy. The funding organization was not involved at any stage of the design or conduct of the study; collection, management, analysis, or interpretation of the data; or final approval of the article.
Disclosure: H.E. is affiliated with the Department of Healthcare Quality Assessment at the University of Tokyo. The department is a social collaboration department supported by the National Clinical Database, Johnson & Johnson K.K., Nipro Corporation, and Intuitive Surgical Sàrl. The remaining authors have no conflicts of interest to disclose.
T.M. and H.E. had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. T.M., I.T., S.H., S.M., K.T., J.K., K.K., Y.K., S.Y., and T.N. concept and design. All authors acquisition, analysis, or interpretation of data. T.M. and H.E. drafting of the manuscript. All authors critical revision of the manuscript for important intellectual content. H.E. statistical analysis. K.H., T.T., T.M., M.W., and Y.K. administrative, technical, or material support. I.T., S.H., M.W., S.Y., and T.N supervision.
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal’s Web site (www.annalsofsurgery.com).
REFERENCES
- 1.Sylla P, Rattner DW, Delgado S, et al. NOTES transanal rectal cancer resection using transanal endoscopic microsurgery and laparoscopic assistance. Surg Endosc. 2010;24:1205–1210. [DOI] [PubMed] [Google Scholar]
- 2.van Oostendorp SE, Belgers HJ, Bootsma BT, et al. Locoregional recurrences after transanal total mesorectal excision of rectal cancer during implementation. Br J Surg. 2020;107:1211–1220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wasmuth HH, Faerden AE, Myklebust T, et al. Transanal total mesorectal excision for rectal cancer has been suspended in Norway. Br J Surg. 2020;107:121–130. [DOI] [PubMed] [Google Scholar]
- 4.Caycedo-Marulanda A, Lee L, Chadi SA, et al. ; Canadian taTME Expert Collaboration. Association of transanal total mesorectal excision with local recurrence of rectal cancer. JAMA Netw Open. 2021;4:e2036330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Roodbeen SX, Penna M, van Dieren S, et al. ; International TaTME Registry Collaborative. Local recurrence and disease-free survival after transanal total mesorectal excision: results from the International TaTME Registry. J Natl Compr Canc Netw. 2021;19:1232–1240. [DOI] [PubMed] [Google Scholar]
- 6.Roodbeen SX, Spinelli A, Bemelman WA, et al. Local recurrence after transanal total mesorectal excision for rectal cancer: a multicenter cohort study. Ann Surg. 2021;274:359–366. [DOI] [PubMed] [Google Scholar]
- 7.Van Oostendorp SE, Belgers HJE, Hol JC, et al. The learning curve of transanal total mesorectal excision for rectal cancer is associated with local recurrence: results from a multicentre external audit. Colorectal Dis. 2021;23:2020–2029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fleshman J, Branda ME, Sargent DJ, et al. Disease-free survival and local recurrence for laparoscopic resection compared with open resection of stage II to III rectal cancer: follow-up results of the ACOSOG Z6051 randomized controlled trial. Ann Surg. 2019;269:589–595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Stevenson ARL, Solomon MJ, Brown CSB, et al. ; Australasian Gastro-Intestinal Trials Group (AGITG) ALaCaRT investigators. Disease-free survival and local recurrence after laparoscopic-assisted resection or open resection for rectal cancer: the australasian laparoscopic cancer of the rectum randomized clinical trial. Ann Surg. 2019;269:596–602. [DOI] [PubMed] [Google Scholar]
- 10.de Lacy AM, Rattner DW, Adelsdorfer C, et al. Transanal natural orifice transluminal endoscopic surgery (NOTES) rectal resection: “down-to-up” total mesorectal excision (TME)--short-term outcomes in the first 20 cases. Surg Endosc. 2013;27:3165–3172. [DOI] [PubMed] [Google Scholar]
- 11.Saito N, Moriya Y, Shirouzu K, et al. Intersphincteric resection in patients with very low rectal cancer: a review of the Japanese experience. Dis Colon Rectum. 2006;49(10 Suppl):S13–S22. [DOI] [PubMed] [Google Scholar]
- 12.Saito N, Ono M, Sugito M, et al. Early results of intersphincteric resection for patients with very low rectal cancer: an active approach to avoid a permanent colostomy. Dis Colon Rectum. 2004;47:459–466. [DOI] [PubMed] [Google Scholar]
- 13.Kitaguchi D, Wakabayashi M, Hasegawa H, et al. Single-stapling technique versus hand-sewn anastomosis in inter-sphincteric resection with transanal total mesorectal excision (Super SST): protocol for a multicentre randomized clinical trial. BJS Open. 2023;7:zrac160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Matsuda T, Yamashita K, Hasegawa H, et al. Intersphincteric resection for rectal cancer using a transanal minimally invasive approach. Dis Colon Rectum. 2022;65:e175. [DOI] [PubMed] [Google Scholar]
- 15.Weiser MR. AJCC 8th edition: colorectal cancer. Ann Surg Oncol. 2018;25:1454–1455. [DOI] [PubMed] [Google Scholar]
- 16.Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg. 2004;240:205–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Japanese Society for Cancer of the Colon and Rectum. Japanese classification of colorectal, appendiceal, and anal carcinoma: the 3d English edition [secondary publication]. J Anus Rectum Colon. 2019;3:175–195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hashiguchi Y, Muro K, Saito Y, et al. ; Japanese Society for Cancer of the Colon and Rectum. Japanese Society for Cancer of the Colon and Rectum (JSCCR) guidelines 2019 for the treatment of colorectal cancer. Int J Clin Oncol. 2020;25:1–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.van Buuren S, Groothuis-Oudshoorn K. mice: Multivariate imputation by chained equations in R. J Stat Softw. 2011;45:9. [Google Scholar]
- 20.van Buuren S. Flexible Imputation of Missing Data. 2nd ed. Vol. 21. Chapman and Hall/CRC;2018. [Google Scholar]
- 21.Hohenberger W, Merkel S, Matzel K, et al. The influence of abdomino-peranal (intersphincteric) resection of lower third rectal carcinoma on the rates of sphincter preservation and locoregional recurrence. Colorectal Dis. 2006;8:23–33. [DOI] [PubMed] [Google Scholar]
- 22.Piozzi GN, Park H, Lee TH, et al. Risk factors for local recurrence and long term survival after minimally invasive intersphincteric resection for very low rectal cancer: multivariate analysis in 161 patients. Eur J Surg Oncol. 2021;47:2069–2077. [DOI] [PubMed] [Google Scholar]
- 23.Ito M. ISR for T1-2 low rectal cancer: a Japanese approach. Clin Colon Rectal Surg. 2020;33:361–365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fields AC, Scully RE, Saadat LV, et al. Oncologic outcomes for low rectal adenocarcinoma following low anterior resection with coloanal anastomosis versus abdominoperineal resection: a national cancer database propensity matched analysis. Int J Colorectal Dis. 2019;34:843–848. [DOI] [PubMed] [Google Scholar]
- 25.van Leersum N, Martijnse I, den Dulk M, et al. Differences in circumferential resection margin involvement after abdominoperineal excision and low anterior resection no longer significant. Ann Surg. 2014;259:1150–1155. [DOI] [PubMed] [Google Scholar]
- 26.Wibe A, Syse A, Andersen E, et al. Oncological outcomes after total mesorectal excision for cure for cancer of the lower rectum: anterior vs abdominoperineal resection. Dis Colon Rectum. 2004;47:48–58. [DOI] [PubMed] [Google Scholar]
- 27.Holm T, Ljung A, Häggmark T, et al. Extended abdominoperineal resection with gluteus maximus flap reconstruction of the pelvic floor for rectal cancer. Br J Surg. 2007;94:232–238. [DOI] [PubMed] [Google Scholar]
- 28.Mège D, de Chaisemartin C, Régis-Marigny L, et al. Supine bottom-up extralevator abdominoperineal excision for anorectal adenocarcinoma is not inferior to standard approach and may be thus safely performed. Surg Endosc. 2023;37:5226–5235. [DOI] [PubMed] [Google Scholar]
- 29.Jeong SY, Park JW, Nam BH, et al. Open versus laparoscopic surgery for mid-rectal or low-rectal cancer after neoadjuvant chemoradiotherapy (COREAN trial): survival outcomes of an open-label, non-inferiority, randomised controlled trial. Lancet Oncol. 2014;15:767–774. [DOI] [PubMed] [Google Scholar]
- 30.van der Pas MH, Haglind E, Cuesta MA, et al. ; COlorectal cancer Laparoscopic or Open Resection II (COLOR II) Study Group. Laparoscopic versus open surgery for rectal cancer (COLOR II): short-term outcomes of a randomised, phase 3 trial. Lancet Oncol. 2013;14:210–218. [DOI] [PubMed] [Google Scholar]
- 31.Sylla P, Knol JJ, D’Andrea AP, et al. ; International taTME Urethral Injury Collaborative. Urethral injury and other urologic injuries during transanal total mesorectal excision: an international collaborative study. Ann Surg. 2021;274:e115–e125. [DOI] [PubMed] [Google Scholar]
- 32.Larsen SG, Pfeffer F, Kørner H; Norwegian Colorectal Cancer Group. Norwegian moratorium on transanal total mesorectal excision. Br J Surg. 2019;106:1120–1121. [DOI] [PubMed] [Google Scholar]
- 33.Koedam TWA, Veltcamp Helbach M, van de Ven PM, et al. Transanal total mesorectal excision for rectal cancer: evaluation of the learning curve. Tech Coloproctol. 2018;22:279–287. [DOI] [PubMed] [Google Scholar]
- 34.Lee L, Kelly J, Nassif GJ, et al. Defining the learning curve for transanal total mesorectal excision for rectal adenocarcinoma. Surg Endosc. 2020;34:1534–1542. [DOI] [PubMed] [Google Scholar]
- 35.Matsuda T, Ryuichiro S, Hasegawa H, et al. Learning curve for transanal total mesorectal excision for low rectal malignancy. J Am Coll Surg. 2023;236:1054–1063. [DOI] [PubMed] [Google Scholar]
- 36.Deijen CL, Velthuis S, Tsai A, et al. COLOR III: a multicentre randomised clinical trial comparing transanal TME versus laparoscopic TME for mid and low rectal cancer. Surg Endosc. 2016;30:3210–3215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lelong B, de Chaisemartin C, Meillat H, et al. ; French Research Group of Rectal Cancer Surgery (GRECCAR). A multicentre randomised controlled trial to evaluate the efficacy, morbidity and functional outcome of endoscopic transanal proctectomy versus laparoscopic proctectomy for low-lying rectal cancer (ETAP-GRECCAR 11 TRIAL): rationale and design. BMC Cancer. 2017;17:253. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.