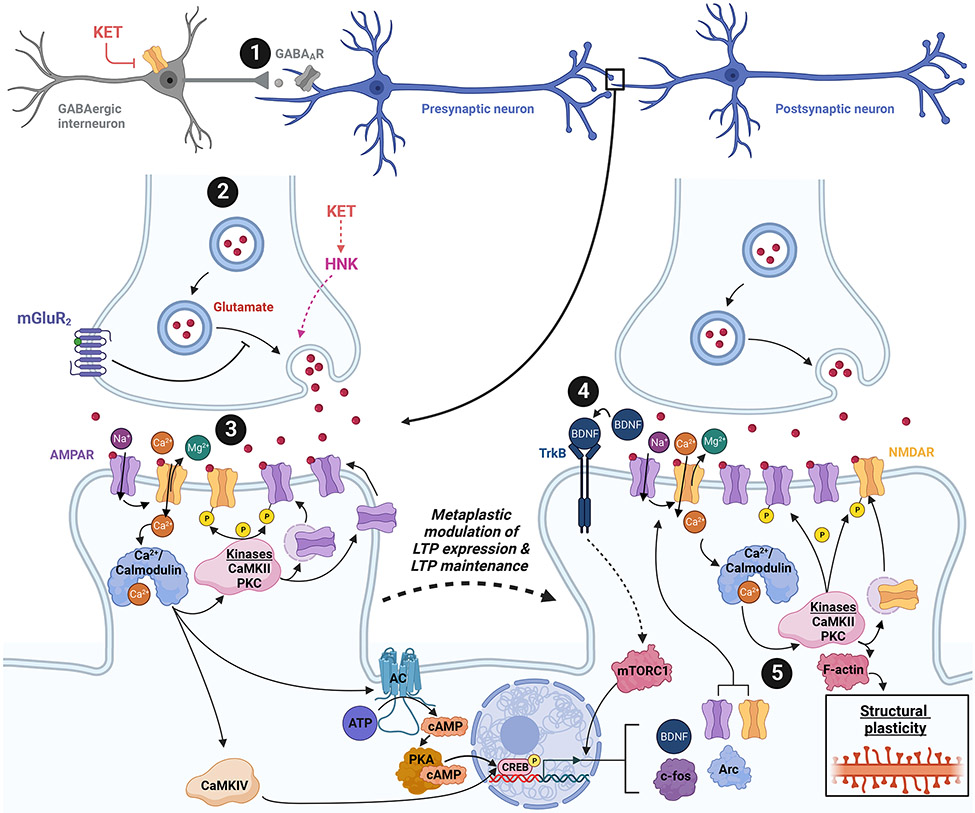
Figure 3. Strategies for targeting metaplastic mechanisms.
Results from preclinical studies suggest that the antidepressant-like effects of ketamine coincide with sustained, beneficial metaplasticity in brain regions implicated in the pathophysiology of depression. Numerous strategies can be used in envisioning ketamine mimetics that may leverage metaplastic mechanisms (metaplastogens) in a manner that coincides with rapid and prolonged antidepressant effects. 1. Disinhibition of glutamatergic neuron activity: Ketamine may work as an antidepressant by disinhibiting glutamatergic neurons via preferential inhibition of NMDARs localized to interneurons [17, 121]. Sustained metaplasticity and long-lasting antidepressant effects are observed after ketamine treatment. Administration of a subanesthetic dose of ketamine is proposed to preferentially block NMDARs localized to interneurons to disinhibit principal cell activity. Reducing the tone of GABAergic activity via negative allosteric modulation (NAM) may also yield similar results (e.g., α5 GABA NAM MRK-016 [11, 211]). 2. Increase probability of glutamate release: Hepatic metabolism of ketamine produces norketamine and, subsequently, hydroxynorketamines. (2R,6R)-HNK has been shown to rapidly enhance glutamatergic transmission via increased probability of glutamate release, followed by sustained metaplasticity and persisting antidepressant-like effects [3]. Inhibition of the glutamate autoreceptor mGluR2 has also been proposed to enhance the probability of glutamate release, augment glutamatergic transmission, facilitate sustained changes in the capacity for synaptic plasticity, and induce prolonged antidepressant-like effects [212]. 3. Augmentation of glutamatergic receptor activity: Direct activation of NMDARs via positive allosteric modulation promotes enhanced glutamatergic transmission, alters the threshold for LTP formation, and results in enduring antidepressant-like effects. Evidence also suggests sustained metaplasticity and antidepressant effects detected after ketamine administration require NMDAR activation [139]. 4. Increase neurotrophic signaling: Ketamine and numerous metaplasticity-engaging putative ketamine mimetics converge around a mechanism that increases neurotrophic signaling (i.e., BDNF-TrkB-mTORC1), suggesting that targeting antidepressant-relevant metaplastic mechanisms to facilitate neurotrophic factor production may be a viable route for designing novel therapeutics. 5. Enhance or prolong mechanisms underlying the expression or maintenance of potentiated synaptic efficacy: The metaplasticity observed after treatment with ketamine and putative ketamine mimetics alters the duration, direction, or magnitude of synaptic plasticity, suggesting that developing therapeutics that engage metaplastic mechanisms along a pathway that converges with canonical NMDAR activation-dependent LTP to modulate mediators of LTP maintenance is a route that may yield exciting, novel treatment modalities for depression. Abbreviations: AC, adenylyl cyclase; AMPAR, α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptor; ATP, adenosine triphosphate; BDNF, brain-derived neurotrophic factor; CaMKII, Ca2+-calmodulin-dependent protein kinase II; CaMKII, Ca2+-calmodulin-dependent protein kinase IV; cAMP, cyclic adenosine monophosphate; CREB, cAMP response element-binding protein; GABA, γ-aminobutyric acid; GABAAR, GABAA receptor; HNK, (2R,6R)-hydroxynorketamine; KET, (R,S)-ketamine; LTP, long-term potentiation; mGluR2, metabotropic glutamate receptor subtype 2; mTORC1, mechanistic target of rapamycin complex 1; NMDAR, N-methyl-D-aspartate receptor; PKA, protein kinase A; PKC, protein kinase C; TrkB, tropomyosin receptor kinase B. Created with Biorender.com