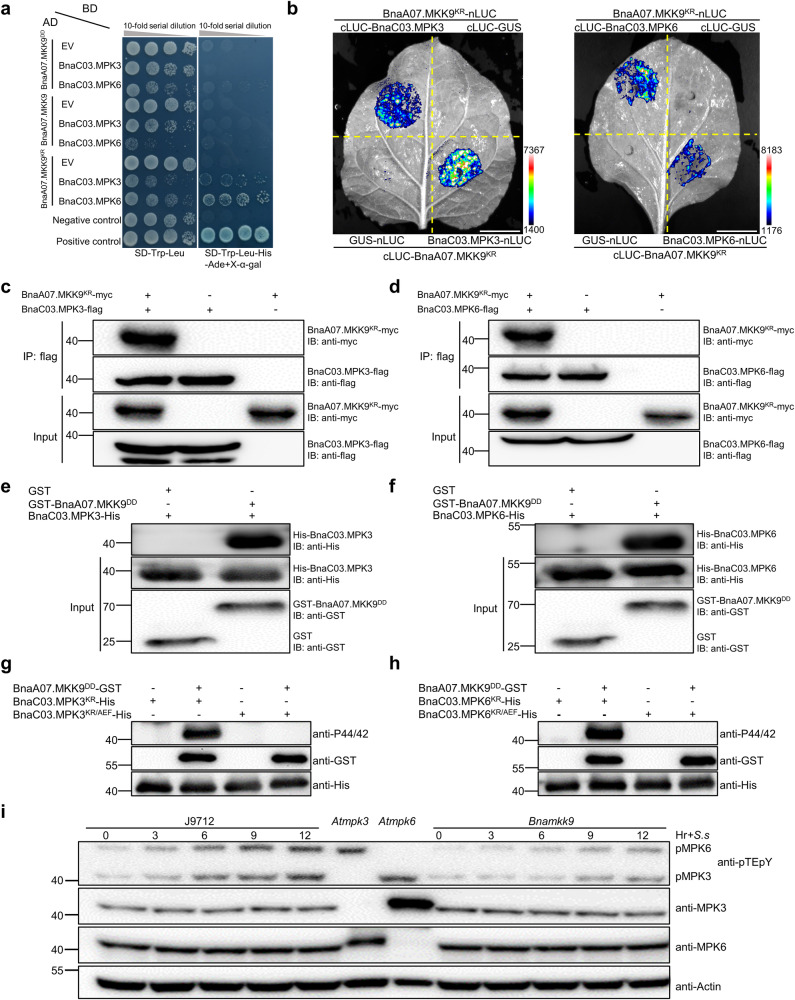
Fig. 4. BnaA07.MKK9 interacts with and phosphorylates BnaMPK3/6 in vitro and in vivo.
a Yeast two-hybrid analysis of the interaction between BnaA07.MKK9KR and BnaC03.MPK3/BnaC03.MPK6. Yeast cells were grown on synthetic defined (SD)-Leu-Trp or SD-Leu-Trp-His-Ade/X-α-Gal medium. BD DNA-binding domain, AD activation domain. BD-53/AD-RecT was the positive control. BD-Lam/AD-RecT was the negative control. b Luciferase complementation imaging in N. benthamiana cells confirmed the interaction between BnaC03.MPK3/BnaC03.MPK6 and BnaA07.MKK9KR, with luciferase signals recorded at 40 h post-infiltration (hpf), scale bar: 1 cm. c, d Co-immunoprecipitation assays using anti-flag beads on protein extracts from N. benthamiana leaves showed the association of BnaA07.MKK9KR with BnaC03.MPK3/6. Western blotting with anti-flag and anti-myc antibodies was conducted on the input and immunoprecipitated proteins, respectively. e, f In vitro pull-down assays further revealed direct interactions between GST-tagged BnaA07.MKK9DD and His-tagged BnaC03.MPK3/BnaC03.MPK6, which were pulled down with anti-GST antibodies and analyzed by immunoblotting (IB) with anti-GST and anti-His antibodies, respectively. g, h Kinase assays conducted in vitro indicated that BnaA07.MKK9DD phosphorylates BnaC03.MPK3/BnaC03.MPK6, with phosphorylation detected by IB using the anti-pTEpY antibody and the input proteins detected with anti-GST and anti-His antibodies. i In Bnamkk9 mutants, phosphorylation of BnaMPK3/6 was reduced compared to J9712 controls after S. sclerotiorum inoculation, with phosphorylation detected by IB with the anti-pTEpY antibody and protein levels detected with anti-BnaMPK6 and anti-BnaMPK3 antibodies. Actin served as the loading control, with Atmpk3 and Atmpk6 mutants providing a reference for the phosphorylation bands of MPK6 and MPK3. Hr+S.s represents the time, in hours, at which inoculation with S. sclerotiorum was conducted. In (c–i), the molecular mass markers in kilodaltons are shown on the left. All experiments were repeated three times, yielding consistent results. Source data are provided as a Source Data file.