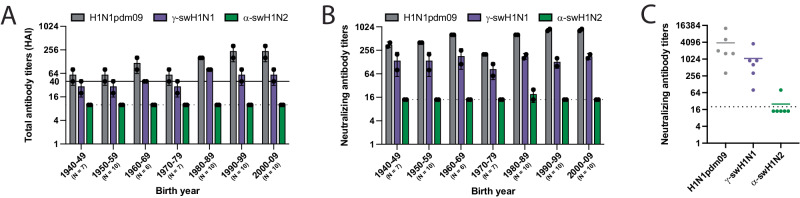
Fig. 3. Cross-reactivity of human sera to swine γ-H1N1 and α-H1N2 influenza viruses.
Pooled sera from the indicated number of humans for each decade of birth were tested for antibodies to H1N1pdm09 (red bars), γ-swH1N1 (purple bars), and α-swH1N2 (green bars) by HAI (A) and neutralization (B) assay. Data are presented as mean values +/− standard deviation (SD) from two biological replicates. Solid line in A indicates an HAI titer of 40, which corresponds to a 50% reduction in the risk of influenza virus infection. C Sera from individuals (N = 6) vaccinated in October 2021 (14 to 21 days post-vaccination) were assessed for cross-reactive neutralizing antibodies. Each dot represents an individual sample and is an average of 2 technical replicates. The colored lines represent the mean values between all the individual biological samples. For A–C, dashed lines indicate the limit of detection for each assay.