Fig. 3. Comparison of CAR-T Cardiotoxicities for Diffuse Large B-Cell Lymphoma (DLBCL).
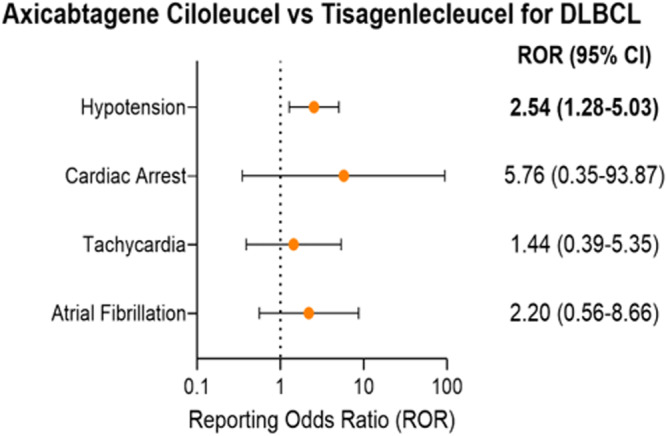
Reporting odds ratios were compared for significant adverse reactions between tisagenlecleucel (N = 237 reports) and axicabtagene ciloleucel (N = 42 reports) for DLBCL patients. Significant adverse reactions include atrial fibrillation (n = 8 reports [tisagenlecleucel] and n = 3 reports [axicabtagene ciloleucel]), tachycardia (n = 12 reports [tisagenlecleucel] and n = 3 reports [axicabtagene ciloleucel]), cardiac arrest (n = 1 reports [tisagenlecleucel] and n = 1 reports [axicabtagene ciloleucel]), and hypotension (n = 54 reports [tisagenlecleucel] and n = 18 reports [axicabtagene ciloleucel]). Error bars denote 95% confidence intervals.
