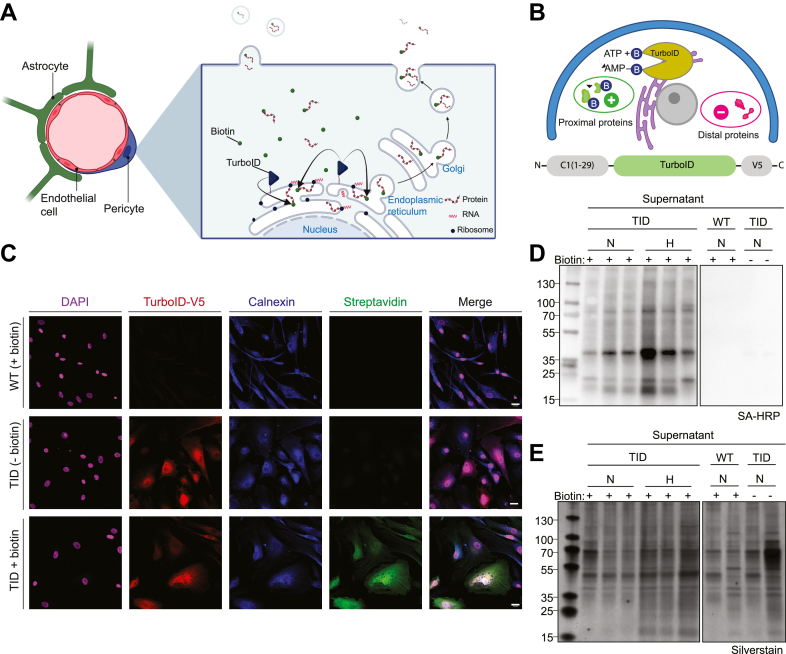
Fig. 1.
TurboID biotinylates intracellular and secreted proteins in pericyte monocultures.A, illustration of TurboID biotin-labeling of translated proteins at the ER and the conventional secretory pathways with ER-Golgi trafficking and/or release of extracellular vesicles. B, illustration of the TurboID construct with the CYP-450 – C1(1–29) ER-anchoring domain at the N-terminal and the V5-tag at the C-terminal and its functionality as a biotin labeling enzyme within ∼20 nm of proximal proteins. C, TurboID-pericytes treated with 500 μM biotin or negative controls including TurboID-pericytes with omitted biotin or WT-pericytes with omitted TurboID ligase were stained for anti-V5 verifying TurboID expression, calnexin marking the ER, and streptavidin-488 for biotin-labeling. D, Western blot of supernatant samples using SA-HRP after 24 h of either normoxic (N) or hypoxic (H) incubation. E, total protein content from the supernatant samples were analyzed with silver stain. Scale bar represents 25 μm. CYP-450, cytochrome P450; ER, endoplasmic reticulum; SA-HRP, streptavidin-horse radish peroxidase; TID, TurboID.