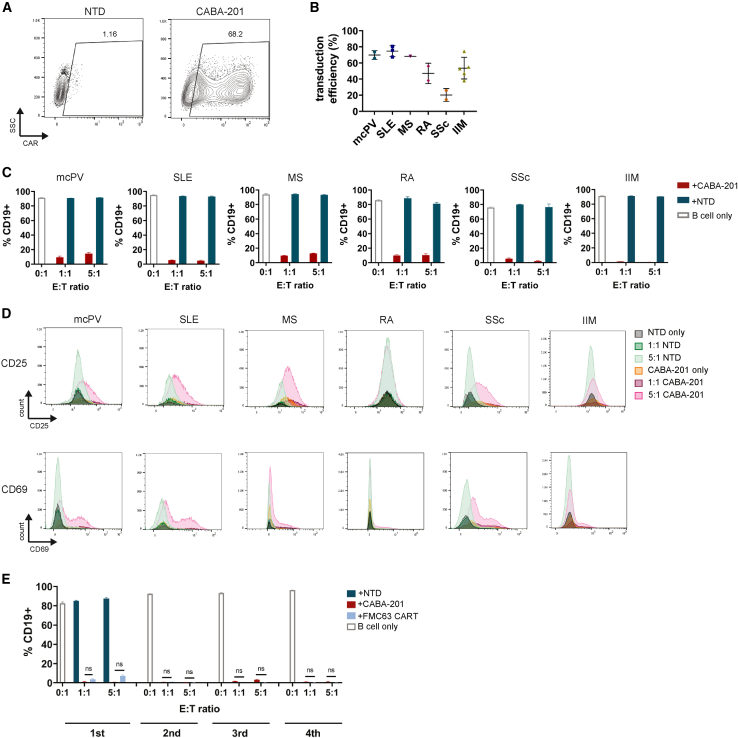
Figure 4.
Cytotoxicity and activation of CABA-201 generated from patient T cells against autologous matched target B cells
(A) Representative dot plots showing primary human T cells isolated from a multiple sclerosis (MS) patient expressing CABA19-IC78 CARs (CABA-201). (B) Summary graph showing transduction efficiency of primary T cells from multiple autoimmune disease patients. mcPV, mucocutaneous pemphigus vulgaris; SLE, systemic lupus erythematosus; RA, rheumatoid arthritis; SSc, systemic sclerosis; IIM, idiopathic inflammatory myositis. Mean ± SD is shown for each disease. (C) Effector T cells (CABA-201 or NTD T cells) generated from mcPV, SLE, MS, RA, SSc, and IIM donors were co-cultured with matched B cells isolated from the same patient at the indicated E:T ratios for 24 h. The percentage of CD19+cells is shown for each representative matched donor pair. Each bar represents mean ± SD of triplicates. (D) Representative histograms of CD25 (upper panel) and CD69 (lower panel) surface expression on effector T cells are shown for each representative matched donor pair following co-culture. 1:1 = E:T ratio of 1:1, and 5:1 = E:T ratio of 5:1. Experiments were repeated six times independently. (E) Effector T cells (CABA-201, FMC63 CAR T, or NTD T cells) manufactured from the SLE donor were co-cultured with matched B cells isolated from the same patient at the indicated E:T ratios for 24 h, and freshly isolated matched B cells were added every 24 h for three more rounds. NTD T cells were only included in the 1st round of co-culture. The percentage of CD19+cells is shown for each round. Each bar represents mean ± SD of triplicates. Statistical differences between CABA-201 and FMC63 CAR T were determined by Mann-Whitney test. ns = not significant.